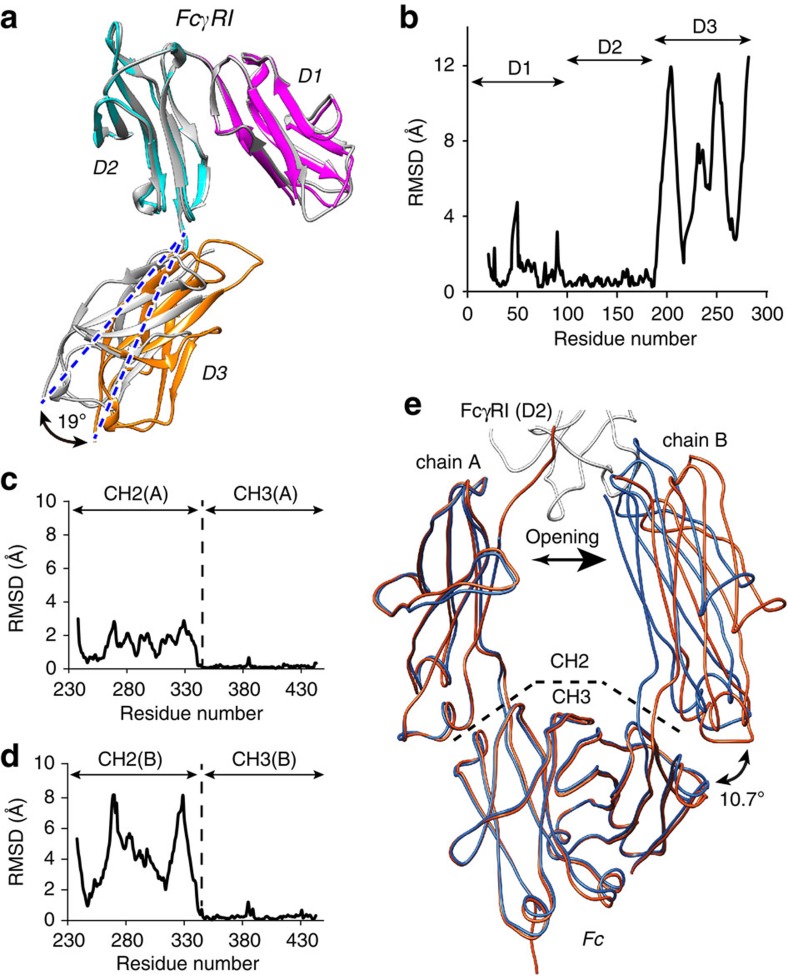
Figure 2. Conformational changes of hFcγRI and Fc upon binding.
(a) Superposition of crystal structures of hFcγRI in the bound and unbound states. Domains D1, D2 and D3 of hFcγRI in the bound state are coloured in magenta, cyan and orange, respectively. Unbound hFcγRI is shown in light grey. The coordinates of unbound hFcγRI were retrieved from the PDB (entry code 3RJD). The rotation angle of domain D3 with respect to domains D1-D2 was calculated with DYNDOM61. (b) RMSD plot of bound and unbound hFcγRI. (c) RMSD plot of residues of chain A of Fc in the bound and unbound conformations. (d) RMSD plot of chain B of Fc. (e) Superposition of Fc in the unbound (blue ribbon) and bound (orange ribbon) conformation. Binding induces the opening of the homodimer as demonstrated by an increase of 9.1 Å in the distance between the α-carbons of Pro239 of chain A and chain B. The rotation angle between domain CH2 and domain CH3 of chain B of Fc increases by 10.7°, as calculated with DYNDOM. The dashed lines separate domain CH2 from CH3. A small portion of hFcγRI is shown at the top of the panel (light grey ribbon).