Abstract
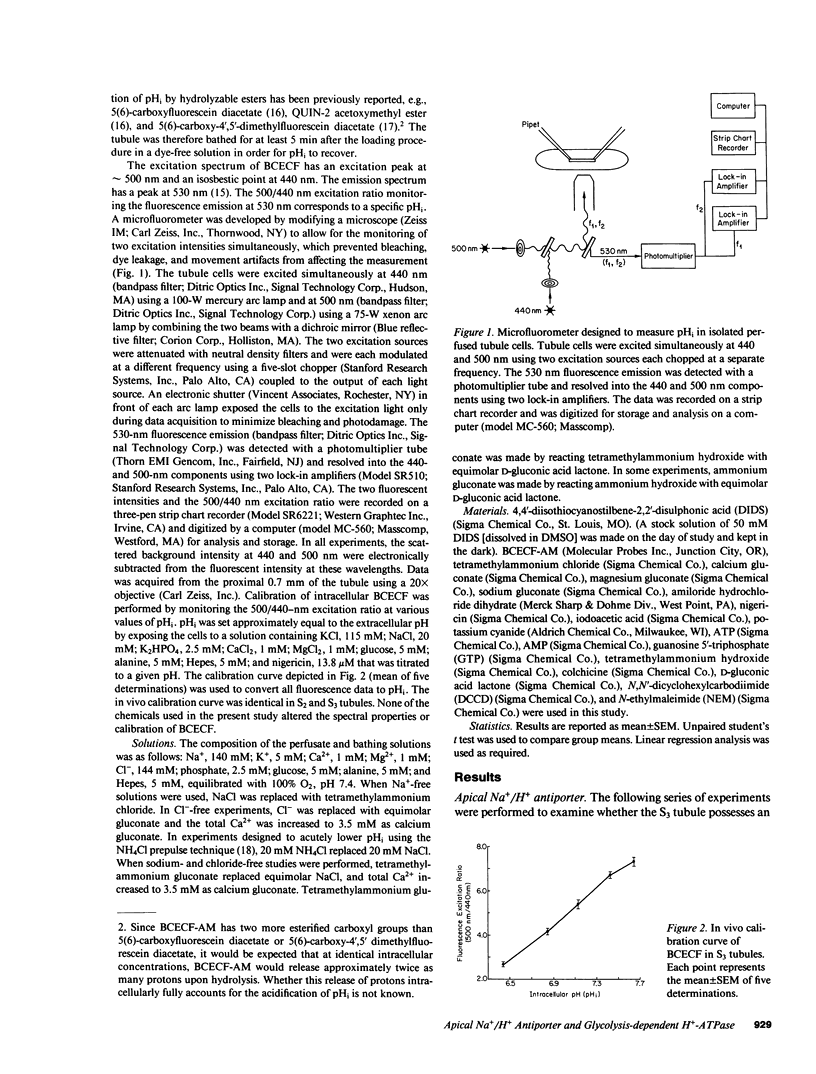
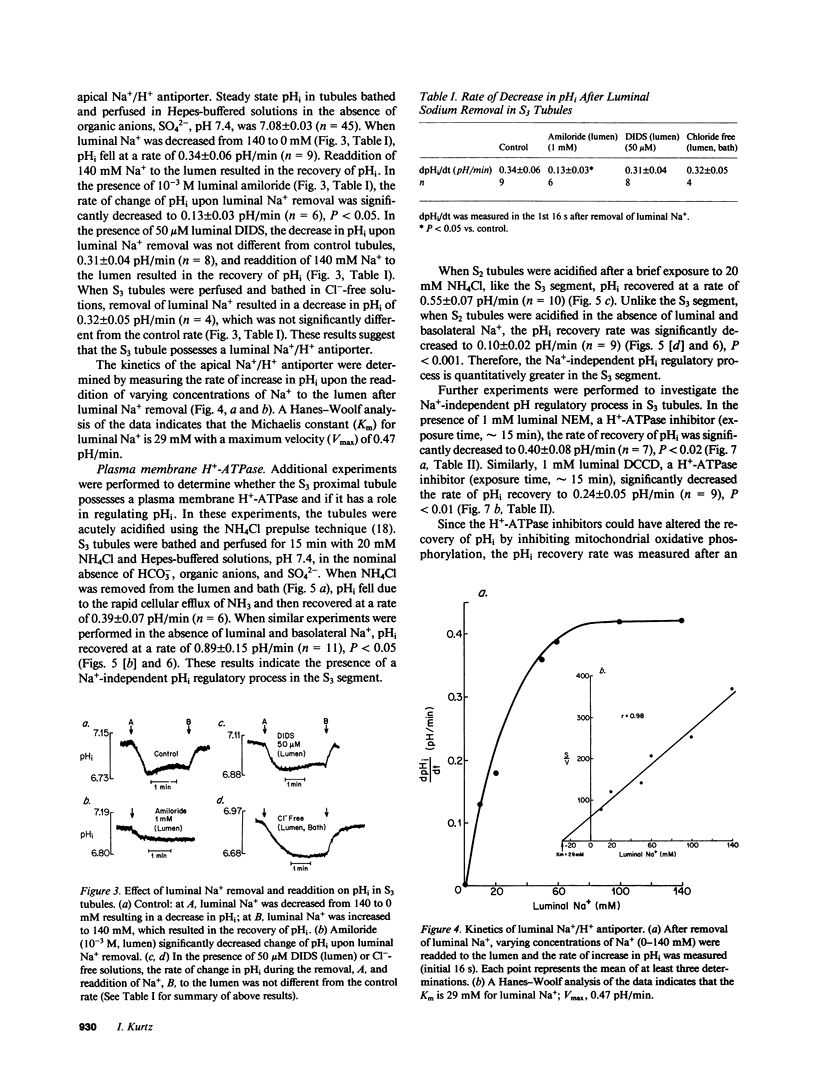
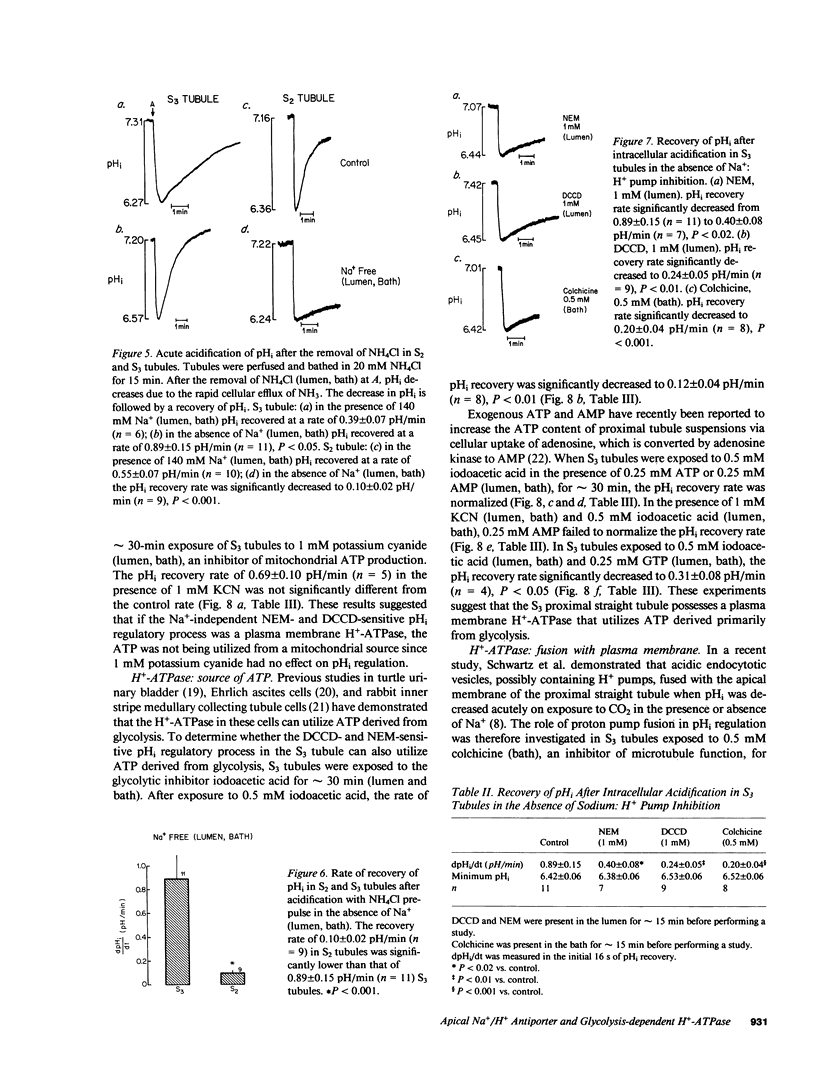
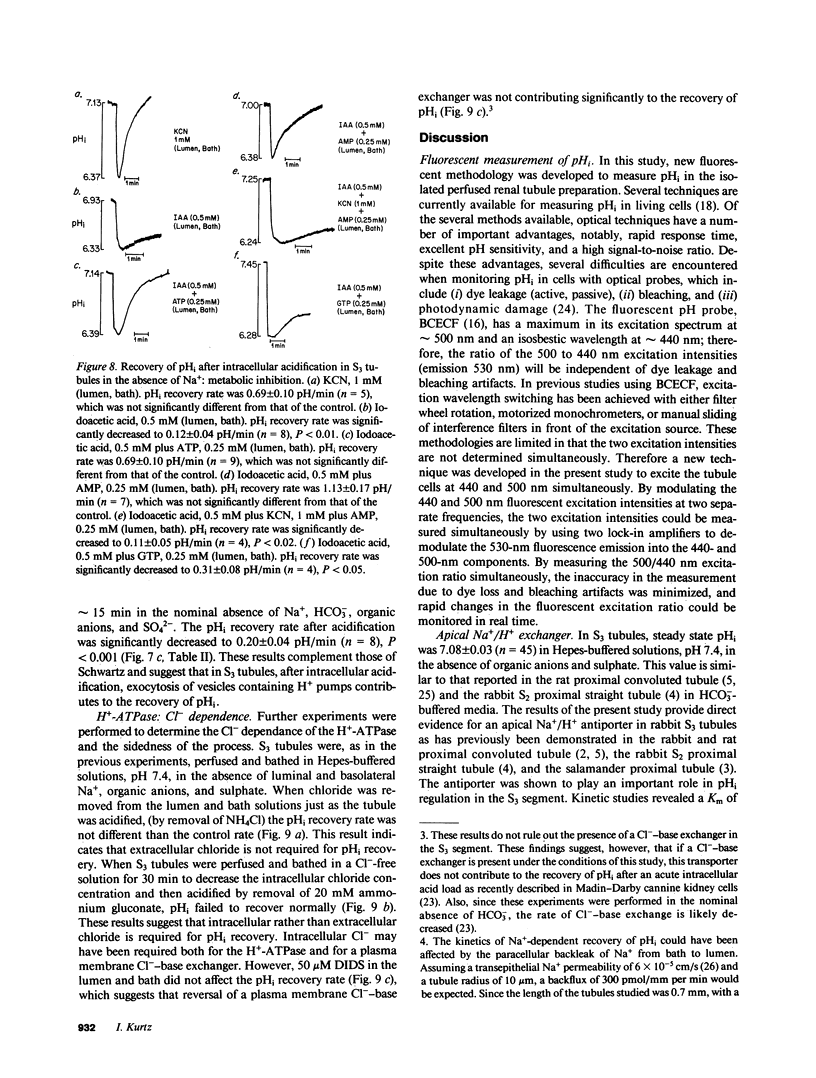
The apical transport processes responsible for proton secretion were studied in the isolated perfused rabbit S3 proximal tubule. Intracellular pH (pHi) was measured with the pH dye, 2',7'-bis(carboxyethyl)-5,6-carboxyfluorescein. Steady state pHi in S3 tubules in nominally HCO3(-)-free solutions was 7.08 +/- 0.03. Removal of Na+ (lumen) caused a decrease in pHi of 0.34 +/- 0.06 pH/min. The decrease in pHi was inhibited 62% by 1 mM amiloride (lumen) and was unaffected by 50 microM 4,4'-diisothiocyanostilbene-2,2'-disulfonic acid (lumen) and Cl- removal (lumen, bath). After a brief exposure to 20 mM NH4Cl, pHi fell by approximately 0.7 and recovered at a rate of 0.89 +/- 0.15 pH/min in the nominal absence of Na+, HCO3-, organic anions, and SO4(2-) (lumen, bath). 1 mM N,N'-dicyclohexylcarbodiimide (lumen), 1 mM N-ethylmaleimide (lumen), 0.5 mM colchicine (bath), and 0.5 mM iodoacetic acid (lumen, bath) inhibited the Na+-independent pHi recovery rate by 73%, 55%, 77%, and 86%, respectively, whereas 1 mM KCN (lumen, bath) did not inhibit pHi recovery. Reduction of intracellular, but not extracellular chloride, also decreased the Na+-independent pHi recovery rate. In conclusion, the S3 proximal tubule has an apical Na+/H+ antiporter with a Michaelis constant for Na+ of 29 mM and a maximum velocity of 0.47 pH/min. S3 tubules also possess a plasma membrane H+-ATPase that can regulate pHi, has a requirement for intracellular chloride, and utilizes ATP derived primarily from glycolysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdelkhalek M. B., Barlet C., Doucet A. Presence of an extramitochondrial anion-stimulated ATPase in the rabbit kidney: localization along the nephron and effect of corticosteroids. J Membr Biol. 1986;89(3):225–240. doi: 10.1007/BF01870666. [DOI] [PubMed] [Google Scholar]
- Alpern R. J., Chambers M. Cell pH in the rat proximal convoluted tubule. Regulation by luminal and peritubular pH and sodium concentration. J Clin Invest. 1986 Aug;78(2):502–510. doi: 10.1172/JCI112602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R. S., Bader J. P. Studies on the relationship between glycolysis and (Na+ + K+)-ATPase in cultured cells. Biochim Biophys Acta. 1984 Aug 17;804(4):419–426. doi: 10.1016/0167-4889(84)90069-7. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S., Mutz B. F. Evidence for a DCCD-sensitive component of proximal bicarbonate reabsorption. Am J Physiol. 1985 Nov;249(5 Pt 2):F636–F644. doi: 10.1152/ajprenal.1985.249.5.F636. [DOI] [PubMed] [Google Scholar]
- Bichara M., Paillard M., Leviel F., Prigent A., Gardin J. P. Na:H exchange and the primary H pump in the proximal tubule. Am J Physiol. 1983 Feb;244(2):F165–F171. doi: 10.1152/ajprenal.1983.244.2.F165. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983 Jan;81(1):29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg M. B. Perfusion of isolated renal tubules. Yale J Biol Med. 1972 Jun-Aug;45(3-4):321–326. [PMC free article] [PubMed] [Google Scholar]
- Chaillet J. R., Boron W. F. Intracellular calibration of a pH-sensitive dye in isolated, perfused salamander proximal tubules. J Gen Physiol. 1985 Dec;86(6):765–794. doi: 10.1085/jgp.86.6.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaillet J. R., Lopes A. G., Boron W. F. Basolateral Na-H exchange in the rabbit cortical collecting tubule. J Gen Physiol. 1985 Dec;86(6):795–812. doi: 10.1085/jgp.86.6.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A., Sachs G., Schafer J. A. Evidence for activation of an active electrogenic proton pump in Ehrlich ascites tumor cells during glycolysis. J Membr Biol. 1981;61(3):143–153. doi: 10.1007/BF01870520. [DOI] [PubMed] [Google Scholar]
- Jacobsen C., Kragh-Hansen U., Sheikh M. I. Na+-H+ exchange in luminal-membrane vesicles from rabbit proximal convoluted and straight tubules in response to metabolic acidosis. Biochem J. 1986 Oct 15;239(2):411–416. doi: 10.1042/bj2390411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinne-Saffran E., Beauwens R., Kinne R. An ATP-driven proton pump in brush-border membranes from rat renal cortex. J Membr Biol. 1982;64(1-2):67–76. doi: 10.1007/BF01870769. [DOI] [PubMed] [Google Scholar]
- Knepper M., Burg M. Organization of nephron function. Am J Physiol. 1983 Jun;244(6):F579–F589. doi: 10.1152/ajprenal.1983.244.6.F579. [DOI] [PubMed] [Google Scholar]
- Kragh-Hansen U., Røigaard-Petersen H., Sheikh M. I. Segmental localization of the rabbit renal proximal tubular Na+-H+ exchange system. Am J Physiol. 1985 Nov;249(5 Pt 2):F704–F712. doi: 10.1152/ajprenal.1985.249.5.F704. [DOI] [PubMed] [Google Scholar]
- Kurtz I., Balaban R. S. Fluorescence emission spectroscopy of 1,4-dihydroxyphthalonitrile. A method for determining intracellular pH in cultured cells. Biophys J. 1985 Sep;48(3):499–508. doi: 10.1016/S0006-3495(85)83805-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtz I., Star R., Balaban R. S., Garvin J. L., Knepper M. A. Spontaneous luminal disequilibrium pH in S3 proximal tubules. Role in ammonia and bicarbonate transport. J Clin Invest. 1986 Oct;78(4):989–996. doi: 10.1172/JCI112690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnensmith R. L., Aronson P. S. The plasma membrane sodium-hydrogen exchanger and its role in physiological and pathophysiological processes. Circ Res. 1985 Jun;56(6):773–788. doi: 10.1161/01.res.56.6.773. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Balaban R. S. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981 May;240(5):F357–F371. doi: 10.1152/ajprenal.1981.240.5.F357. [DOI] [PubMed] [Google Scholar]
- Mercer R. W., Dunham P. B. Membrane-bound ATP fuels the Na/K pump. Studies on membrane-bound glycolytic enzymes on inside-out vesicles from human red cell membranes. J Gen Physiol. 1981 Nov;78(5):547–568. doi: 10.1085/jgp.78.5.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink T. J., Tsien R. Y., Pozzan T. Cytoplasmic pH and free Mg2+ in lymphocytes. J Cell Biol. 1982 Oct;95(1):189–196. doi: 10.1083/jcb.95.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Characteristics of the proton pump in rat renal cortical endocytotic vesicles. Am J Physiol. 1986 May;250(5 Pt 2):F817–F826. doi: 10.1152/ajprenal.1986.250.5.F817. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Shigai T., Takeuchi J. Intracellular pH in the isolated perfused rabbit proximal straight tubule. Am J Physiol. 1985 Sep;249(3 Pt 2):F417–F423. doi: 10.1152/ajprenal.1985.249.3.F417. [DOI] [PubMed] [Google Scholar]
- Schwartz G. J. Na+-dependent H+ efflux from proximal tubule: evidence for reversible Na+-H+ exchange. Am J Physiol. 1981 Oct;241(4):F380–F385. doi: 10.1152/ajprenal.1981.241.4.F380. [DOI] [PubMed] [Google Scholar]
- Schwartz J. H., Steinmetz P. R. Metabolic energy and PCO2 as determinants of H+ secretion by turtle urinary bladder. Am J Physiol. 1977 Aug;233(2):F145–F149. doi: 10.1152/ajprenal.1977.233.2.F145. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Nerbonne J., Campos de Carvalho A., Harris A. L., Bennett M. V. Substituted benzyl acetates: a new class of compounds that reduce gap junctional conductance by cytoplasmic acidification. J Cell Biol. 1984 Jul;99(1 Pt 1):174–179. doi: 10.1083/jcb.99.1.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshitomi K., Frömter E. Cell pH of rat renal proximal tubule in vivo and the conductive nature of peritubular HCO3- (OH-) exit. Pflugers Arch. 1984 Nov;402(3):300–305. doi: 10.1007/BF00585513. [DOI] [PubMed] [Google Scholar]
- Zeidel M. L., Silva P., Seifter J. L. Intracellular pH regulation and proton transport by rabbit renal medullary collecting duct cells. Role of plasma membrane proton adenosine triphosphatase. J Clin Invest. 1986 Jan;77(1):113–120. doi: 10.1172/JCI112264. [DOI] [PMC free article] [PubMed] [Google Scholar]


