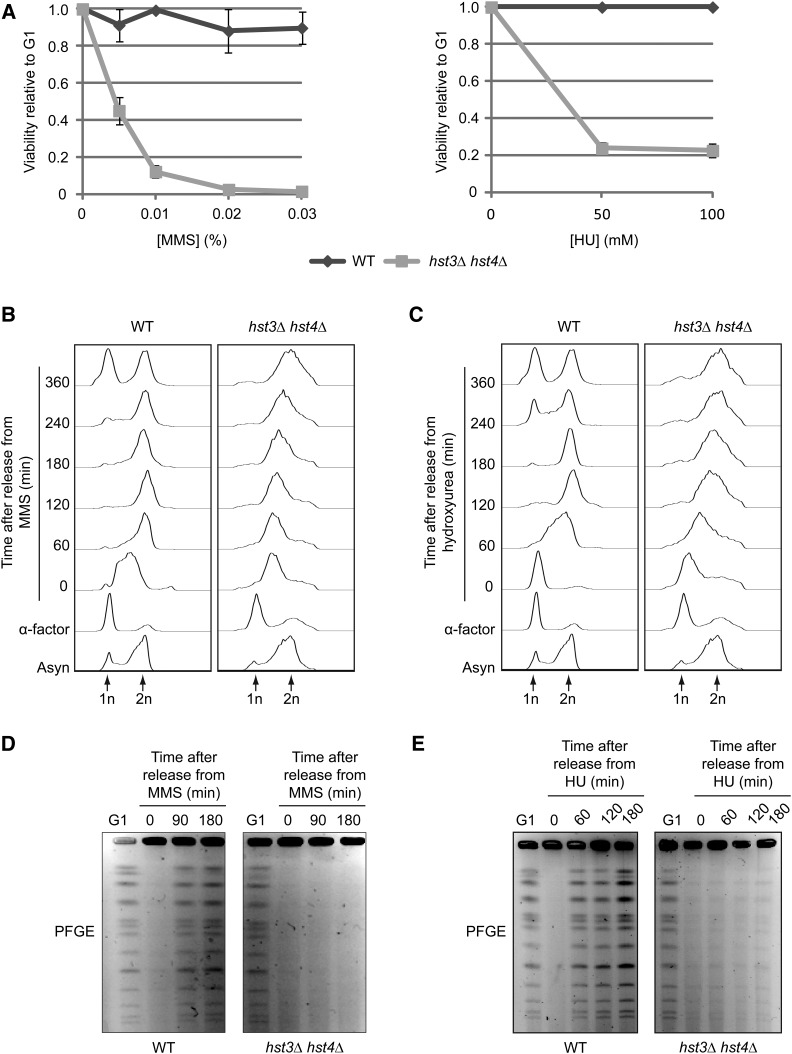
Figure 1.
Transient exposure of hst3Δ hst4Δ cells to MMS or HU causes loss of viability and prevents the completion of DNA replication. (A) hst3∆ hst4∆ cells are sensitive to transient exposure to MMS and HU during S phase. Cells were arrested in G1 and released into the cell cycle in the presence of increasing concentrations of MMS (left panel) or HU (right panel) at 25°. Appropriate dilutions of cells were plated on YPD during G1 arrest and after 90 min of MMS exposure. Viability was defined as the ratio of colonies that arose after MMS or HU treatment to colonies formed by G1-synchronized cells (see Materials and Methods). (B and C) Transient exposure to MMS or HU delays the completion of DNA replication in hst3∆ hst4∆ mutants. Cells were synchronized in G1 with α-factor and released toward S phase in a medium containing 0.03% MMS or 200 mM HU for 90 min. Genotoxic agents then were washed away and inactivated using 2.5% sodium thiosulfate in the case of MMS, and cells were released into fresh medium lacking genotoxins. Samples were processed for cell-cycle analysis by FACS at the indicated time points. Asyn, asynchronous cells. (D and E) hst3∆ hst4∆ mutants cannot complete chromosome duplication after transient exposure to MMS. Cells were arrested in G1 and released into the cell cycle in the presence of 0.03% MMS or 200 mM HU for 1.5 hr. They were washed with YPD (containing 2.5% sodium thiosulfate in the case of MMS) and resuspended in fresh medium lacking genotoxins. Samples were taken at the indicated time points and processed for pulse-field gel electrophoresis.