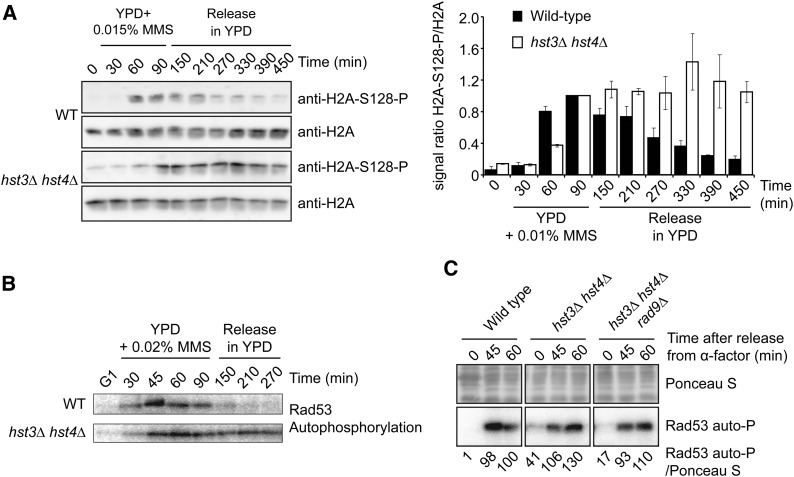
Figure 3.
Persistent activation of DNA damage–induced signaling in hst3∆ hst4∆ mutants exposed to MMS. (A) hst3∆ hst4∆ mutants display persistent phosphorylation of histone H2A serine 128 following transient exposure to MMS. Cells were arrested in G1 and released into the cell cycle in the presence of 0.015% MMS for up to 90 min. Cells were washed in YPD medium containing 2.5% sodium thiosulfate to inactivate MMS and resuspended in fresh YPD without MMS. (Left panel) Aliquots of cells were collected, and whole-cell lysates were analyzed by immunoblotting to detect histone H2A S128 phosphorylation (H2A-S128-P) and nonmodified H2A. (Right panel) H2A-S128-P signals were quantified by densitometry and normalized relative to H2A levels. For both strains, the value of time point “90 min” (end of MMS exposure) was set to 1, and values for other samples were normalized relative to this point. Error bars: standard error of the mean of densitometry values (three loadings of the immunoblot samples). (B) Cells were treated as in A except that 0.02% MMS was used, and autophosphorylation of Rad53 was detected using in situ kinase assay (see Materials and Methods). (C) hst3Δ hst4Δ rad9∆ triple mutants do not display Rad53 activation defects in response to HU-induced replication block. Cells were synchronized in G1 using α-factor and released into YPD medium containing 200 mM HU. Samples were taken at the indicated time points, and Rad53 activity was monitored by in situ Rad53 autophosphorylation assay (Rad53 auto-P). Equal amounts of total protein were loaded for each sample. Rad53 autophosphorylation signals were quantified by densitometry relative to Ponceau S staining. Values were normalized to the “60 min” sample of the WT strain.