Abstract
Background
Ceriporia lacerata, a strain of white-rot fungus isolated from the litter of an invasive plant (Solidago canadensis) in China, was little known about its properties and utilization. In this work, the copper(II) biosorption characteristics of formaldehyde inactivated C. lacerata biomass were examined as a function of initial pH, initial copper(II) concentration and contact time, and the adsorptive equilibrium and kinetics were simulated, too.
Results
The optimum pH was found to be 6.0 at experimental conditions of initial copper(II) concentration 100 mg/L, biomass dose 2 g/L, contact time 12 h, shaking rate 150 r/min and temperature 25°C. Biosorption equilibrium cost about 1 hour at experimental conditions of pH 6.0, initial copper(II) concentration 100 mg/L, C. lacerata dose 2 g/L, shaking rate 150 r/min and temperature 25°C. At optimum pH 6.0, highest copper(II) biosorption amounts were 6.79 and 7.76 mg/g for initial copper(II) concentration of 100 and 200 mg/L, respectively (with other experimental parameters of C. lacerata dose 2 g/L, shaking rate 150 r/min and temperature 25°C). The pseudo second-order adsorptive model gave the best adjustment for copper(II) biosorption kinetics. The equilibrium data fitted very well to both Langmuir and Freundlich adsorptive isotherm models.
Conclusions
Without further acid or alkali treatment for improving adsorption properties, formaldehyde inactivated C. lacerata biomass possesses good biosorption characteristics on copper(II) removal from aqueous solutions.
Keywords: Ceriporia lacerata, Biosorption, Copper, Adsorption isotherm, Kinetics
Background
Heavy metal pollution is an increasing environmental problem of worldwide concern. Reducing heavy metals to environmentally acceptable limits in a cost-effective, easily available and environmental friendly manner becomes more and more urgent [1,2]. Biosorption of heavy metals from wastewaters by pretreated fungal biomass has gained growing acceptance since the 1990s [3,4].
The heavy metal ion biosorption by fungal biomass is based mainly on two mechanisms: covalent bonding with functional groups including carboxyl, hydroxyl, phosphate, amino, sulphydryl, and the result of physicochemical inorganic interactions directed by adsorption phenomena [2,5-7]. Therefore, here are several critical parameters affecting biosorption characteristics, such as pH, pretreatment methods, metal species, initial concentration of solutions, quantity of biomass, contact time [8-10].
Many fungi have been extensively studied and proved to be good biosorbents of heavy metals, such as Rhizopus arrhizus [11-14], Aspergillus spp. [6,15-17], Penicillium spp. [17,18] and Saccharomyces spp. [2,19]. However, white-rot fungi were relatively less reported for their biosorption though they were strong degrader of various xenobiotics and detoxicating materials of contaminated effluents [20,21]. They also possess the capacity of heavy metal biosorption [21].
Ceriporia lacerata is a white-rot fungus first isolated as a new species from white-rotted wood in Japan [22]. Till 2006, only four other reports published about it, referring to its taxonomy, genetics or decomposition [23-25]. Since 2007, C. lacerata has been more widely researched on its clinical significance, wood-decaying effect, metal tolerance and sorption potential and some other characteristics [26-30]. Kim et al. [31] found that the cadmium(II) removal rates by C. lacerata in stationary and shaking cultures were about 7% and 11%, respectively. However, there is so limited information yet available on this species that its other properties need further study. The objectives of this work were to verify the capacity of dead C. lacerata in copper(II) removal under batch conditions, to determine the influences of parameters involved, and to simulate the adsorptive equilibrium and kinetics.
Materials and methods
Preparation of the biomass
Fungus C. lacerata was isolated from the litter of Solidago canadensis (an exotic plant to China) in Pukou, Nanjing, China. It was cultivated at 25°C in 250 mL flasks containing 100 mL liquid medium composed of malt extract (20 g/L), peptone (1 g/L) and dextrose (20 g/L). After about 10 days incubation on a shaker at 150 r/min, C. lacerata mycelium was washed several times with deionized water, and then inactivated by immersion into 1% formaldehyde. After washing, the mycelium was dried at 60°C for 24 hour (h). Finally, dry mycelium was ground and sieved (mesh size < 0.5 mm).
Metal solutions
Copper(II) solutions of 5 to 300 mg/L were obtained by diluting copper(II) stock solution (1 g/L), which was prepared by dissolving CuCl2 · 2H2O (analytical reagent grade, Shanghai Zhenxing Chemical Reagent Factory, China) in deionized water. Solution pH was adjusted with 0.1 mol/L HCl and NaOH and measured by pH meter (PHS-3C, Shanghai Hongyi Instrumentation Co., Ltd, China).
Batch biosorption experiments
Batch biosorption experiments were conducted separately to evaluate the effects of pH, time, initial copper(II) concentration on biosorption of copper ions. All experiments were performed in duplicate, and the mean values were taken as the final results. For every treatment, 0.2 g dead biomass was added into 100 mL of copper(II) solution in 250 mL flask. The flasks were shaken (150 r/min) at 25°C for 12 h. Then, copper(II) solutions were vacuum filtered through Millipore membrane filters (0.45 μm, Shanhai Xingya Purification Material Factory, China). After dilution, initial and equilibrium copper(II) concentrations were determined using an atomic absorption spectrometer (AA320CRT, Shanghai Analytical Instrument Overall Factory, China). The copper(II) biosorption amount was calculated by Eq. (1):
| 1 |
where qe (mg/g) is the amount of copper(II) adsorbed on per gram of biosorbent, V (L) is the volume of copper(II) solution in the flasks, C0 and Ce (mg/L) are the initial and equilibrium copper(II) concentration, respectively, and m (g) is the dry weight of dead C. lacerata biomass.
Experiments to evaluate the effect of pH on biosorption were conducted constantly at pH 2.5 to 7.0, with intervals of 0.5, while initial copper(II) concentration was 100 mg/L.
Experiments to analyze the effect of contact time were operated at optimum pH and copper(II) concentration of 100 mg/L. Samples were harvested at 1/12, 1/6, 1/4, 1/2, 1, 2, 4, 6, 8 and 12 h.
Experiments to analyze the effect of initial sorbate concentration were performed at 5, 10, 25, 50, 75, 100, 200 and 300 mg/L (at optimum pH).
Biosorption kinetics analysis
Kinetic models can simulate the data of contact time experiments. This study was simulated by both the linear first-order Lagergren (Eq. (2)) and pseudo second-order (Eq. (3)) models [6,32-34]:
| 2 |
| 3 |
where k’ (h−1) and kP (g/mg · h) are the first and second-order rate constants, respectively, qe and qt are the amounts of copper entrapped on per gram of biosorbent (mg/g) at equilibrium and time t (h), respectively.
Biosorption isotherm analysis
Experiments to evaluate the effect of initial sorbate concentration were also used for biosorption isotherm studies. Langmuir and Freundlich isotherms were used to simulate the experimental data from the batch system at 25°C.
The linear Langmuir isotherm equation is:
| 4 |
where qmax (maximum possible amount of copper adsorbed on per gram of biosorbent, mg/g) is the monolayer biosorption capacity of the biomass, and KL is the Langmuir adsorption constant (L/mg) [21,35]. The equation of the Freundlich model is:
| 5 |
where KF and n are Freundlich adsorption constants and they respectively indicate adsorption capacity and intensity [10,36].
Results and discussion
Effect of pH on biosorption
Copper(II) biosorption capacity of dead C. lacerata biomass at different pH is shown in Figure 1. Approximately, the biosorption capacity of biomass increased with an increase of pH from 2.5 to 6.0, and then decreased at pH 6.5 and 7.0. Additionally, during the adsorptive process, the pH of equilibrium solution was slightly lower than that of initial solution.
Figure 1.
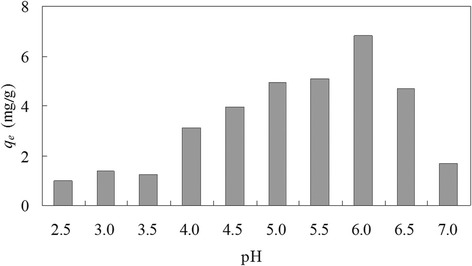
The effect of solution pH on copper biosorption by Ceriporia lacerata. Experimental conditions: initial copper(II) concentration = 100 mg/L, volume of copper(II) solution = 100 mL, Ceriporia lacerata dose = 2 g/L, contact time = 12 h, shaking rate = 150 r/min, temperature = 25°C.
Previous studies showed that pH value of the solution was an important parameter for both solution chemical properties of metals and surface characteristics of biosorbents [37-41]. According to Asmal et al. [42], there are three species of copper present in solution: Cu2+, CuOH+ and Cu(OH)2. At low pH (here maybe from 2.5 to 3.5), H+ ions competed with Cu2+ ions for the biosorption sites, that is protonation of the cell wall components negatively affected the biosorption capacity of dead C. lacerata biomass. However, this effect became less with the increase in pH (from 4.0 to 6.0) owing to that the raise of negative charges density on the cell surface offered more metal binding sites [6,7,15]. At this pH Cu2+ and CuOH+ were more favourable copper species. Therefore, just like the results of some works [6,43-45], we also found a sharp increase in biosorption with a slight increase of pH (at around pH 3.5). At higher pH (≥6.5), precipitation of copper(II) hydroxide occurred and precipitated on surfaces of biomass and bottle wall. Furthermore, all those above suggested that ion-exchange played an important role in biosorption of copper(II) ions by dead C. lacerata.
The optimum pH was 6.0 at which copper(II) biosorption capacity of dead C. lacerata biomass reached 6.79 mg/g. The optimum pH values of different reports on copper(II) biosorption by different biomasses differ quite a bit. Phanerochaete chrysosporium fungal biomasses [7,40] and three species of dead fungal biomasses (Cladosporium cladosporioides, Gliomastix murorum and Bjerkandera sp.) [46] showed the same optimum pH 6.0 for copper(II) removal, while cone biomass of Thuja orientalis showed the optimum pH value to be 7.7 [47] and Chlorella vulgaris algal biomass to be 5.0. The components and structural characteristics of various biomasses are quite diverse, which may be the most important reason for optimum pH differences. There must be other reasons such as different experimental parameters and operating error.
Effect of contact time on biosorption
At optimum pH, the amount of copper(II) adsorbed by dead C. lacerata increased during the biosorption process (Figure 2). This process consisted of two phases: the rapid phase during the first one hour at which biosorption contributed significantly great to adsorptive equilibrium, and the subsequent slower phase when biosorption contributed relatively small. At the end of the rapid phase, the amount of copper(II) biosorption reached 86% of the equilibrium which cost about 1 hour. The copper(II) biosorption decreased at the 2nd and 4th hours might because at that time the slightly decrease of pH resulted in H+ ions competing slightly with Cu2+ and CuOH+ ions for the biosorption sites and/or because of experimental errors, which needs to be further studied.
Figure 2.
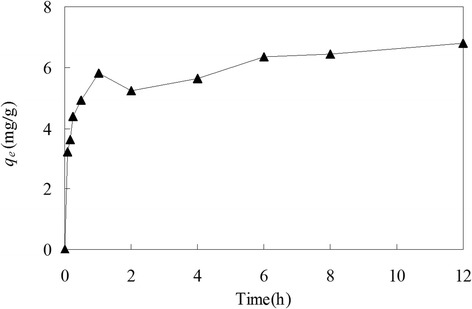
The amount of copper biosorption by Ceriporia lacerata at different contact time. Experimental conditions: pH 6.0, initial copper(II) concentration = 100 mg/L, volume of copper(II) solution = 100 mL, Ceriporia lacerata dose = 2 g/L, shaking rate = 150 r/min, temperature = 25°C.
The equilibrium time of the copper biosorption by fungal biomass is determined by many parameters such as agitation rate of the solution, pretreated methods of the fungal biomass, structural properties and quantity of the biosorbents, the existence of other metal ions and initial copper(II) concentration [21]. Therefore, one species of biosorbent may cost different time (ranging from a few minutes to several hours [7,14,48]) to reach equilibrium under different conditions. One hour as the equilibrium time of copper biosorption in this study was scarcely reported before.
Effect of initial copper(II) concentration on biosorption
The effect of initial copper(II) concentration on biosorption is presented in Figure 3. Approximately, copper biosorption increased with the increase of initial copper(II) ion concentration at the same unprecipitable pH. At pH 6.0, copper biosorption capacity increased sharply from 0.85 mg/g to 6.79 mg/g (Figure 3) while the initial copper(II) concentration was from 5 to 100 mg/L, but this increase became minor if the initial concentration continued to be raised. The highest biosorption capacity was 7.76 mg/g for 200 mg/L at pH 6.0.
Figure 3.
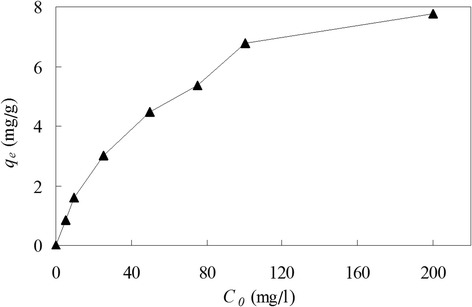
The effect of initial copper(II) concentration on copper biosorption by Ceriporia lacerata at optimum pH 6.0. Experimental conditions: pH 6.0, volume of copper(II) solution = 100 mL, Ceriporia lacerata dose = 2 g/L, contact time = 12 h, shaking rate = 150 r/min, temperature = 25°C.
At pH 6.0, copper(II) precipitated at initial concentrations higher than 300 mg/L. Under the same conditions, in the solutions of higher concentration, there were much more copper(II) ions around the active sites of C. lacerata biomass. Thus the adsorptive process could proceed more sufficiently, and that is why copper(II) adsorptive capacity increased with the increasing of initial concentration.
Biosorption kinetics
Mathematical models could be used for the quantitative description of the kinetic results. There were two kinetic equations very often used to simulate the metal ion adsorptive kinetics: the first-order Lagergren and the pseudo second-order rate equations [6,33;49]. In this study, the former with qe (3.37 mg/g) and R’2 (0.8233) did not well fit experimental data, while the latter fitted the kinetic data with high regression coefficient (RP2, 0.9958) statistically significant (p < 0.05) (Figure 4 and Table 1). As a constant of pseudo second-order rate model, theoretical qe (6.76 mg/g) was very close to the experimental qe value (6.79 mg/g). That may be due to that biosorption is the rate-limiting step involving valence forces through sharing or exchanging electrons between biosorbent and sorbate [49]. In most cases (including this work), the former is applicable during the initial 1/3 or 1/2 hour but does not apply well throughout the whole process [49,50]. The pseudo second-order model was also proved most reliable by many researches [6,33,49,51].
Figure 4.
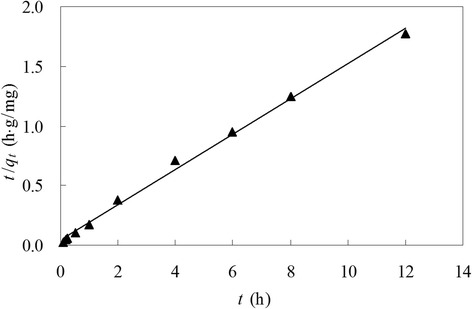
Linear plot of the pseudo second-order equation for copper biosorption by Ceriporia lacerata. Experimental conditions: pH 6.0, initial copper(II) concentration = 100 mg/L, volume of copper(II) solution = 100 mL, Ceriporia lacerata dose = 2 g/L, contact time = 12 h, shaking rate = 150 r/min, temperature = 25°C.
Table 1.
Kinetic model constants for copper biosorption by C . lacerata at pH 6 and 25°C
| Kinetic model | Constants of model | |||
|---|---|---|---|---|
| First-order Lagergren | q e = 3.37 mg/g | k’ = 11.05 × 10−2 h−1 | R’ 2 = 0.8233 | p < 0.05 |
| Pseudo second-order | q e = 6.76 mg/g | k P = 4.68 × 10−4 g/mg · h | R P 2 = 0.9958 | p < 0.05 |
Biosorption isotherms
Biosorption isotherm procedure assumes that all the external biosorption system parameters like pH and ionic strength are constant [52]. The Langmuir model assumes a monolayer adsorption of which energy is constant and no migration of sorbate molecules in the surface plane [2,5,35]. The Freundlich model is an empirical equation based on adsorption on a heterogeneous surface [36]. The former presented the better adjustment for copper(II) adsorption by dead C. lacerata than the latter did (Figure 5). The Langmuir model presented good adjustment with a regression coefficient (RL2) of 0.9979 (p < 0.05), and Freundlich model also gave a not bad adjustment with a RF2 value of 0.9696 (p < 0.05) (Figure 5 and Table 2).
Figure 5.
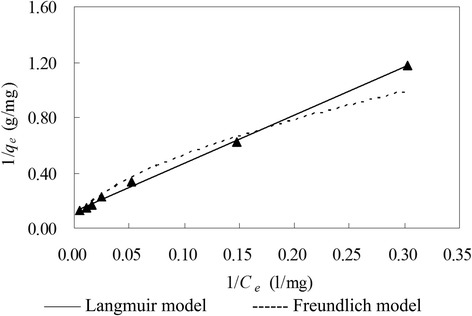
Copper biosorption isotherms by Ceriporia lacerata at pH 6 and 25°C. Experimental conditions: pH 6.0, initial copper(II) concentration = 5–200 mg/L, volume of copper(II) solution = 100 mL, Ceriporia lacerata dose = 2 g/L, contact time = 12 h, shaking rate = 150 r/min, temperature = 25°C.
Table 2.
Isotherm model constants for copper biosorption by C . lacerata at pH 6 and 25°C
| Isotherm models | Constants of models | |||
|---|---|---|---|---|
| Langmuir | q max = 8.31 mg/g | K L = 3.45 × 10−2 mg−1 | R L 2 = 0.9979 | p < 0.05 |
| Freundlich | n = 0.56 | K F = 0.52 | R F 2 = 0.9696 | p < 0.05 |
The Langmuir model showed that the maximum capacity of adsorbing copper(II) was 8.31 mg/g, which was assumed that at pH 6.0, 8.31 mg copper(II) would form a complete monolayer onto the surface of per gram of dead C. lacerata. The Freundlich model had constants of 0.52 for KF value related to the adsorption capacity and 0.56 for n value related to the adsorption intensity.
Comparison of biosorption capacity with other adsorbents
The maximum biosorption amount (qmax) depends on fungal species, pretreating methods, the performed parameters. 8.31 mg/g as the ideal maximum value (qmax) of copper(II) biosorption by dead C. lacerata was resulted at these conditions: initial copper(II) concentration of 100 mg/L, biomass 2 g/L, solution 100 mL, rotation 150 r/min and pH 6.0. The comparison of copper(II) adsorption capacities between dead C. lacerata biomass and other fungal biosorbents is shown in Table 3.
Table 3.
Copper(II) adsorption capacities (calculated from Langmuir constant q max ) and experimental parameters of various fungal biosorbents from the literatures
| Adsorbent | pH | C e (mg/L) | q max (mg/g) | References |
|---|---|---|---|---|
| NaOH-treated Aspergillus niger | 6.0 | 0-10 | 6.35 | [6] |
| NaOH-treated Botrytis cinerea | 5.0 | 5-300 | 20.35 | [53] |
| Hydrochloric acid-treated waste beer yeast | 5.0 | 3.2-44.8 | 1.46 | [54] |
| Immobilized Phanerochaete chrysosporium | 6.0 | 10-500 | 99.85 | [40] |
| Dead Pleurotus pulmonarius (HCHO inactivated) | 4.0 | 5-200 | 6.20 | [10] |
| Dead Schizophyllum commune (HCHO inactivated) | 4.0 | 5-200 | 1.52 | [10] |
| Dead Ceriporia lacerata (HCHO inactivated) | 6.0 | 5-200 | 8.31 | This study |
Pretreatments (taking NaOH-boiling and immobilization as examples) could avail copper(II) ions more functional groups to bind. That may be why formaldehyde inactivated C. lacerata biomass had lower biosorption capacity than those pretreated fungal biosorbents. Compared with other unpretreated fungal adsorbents, however, biosorption capacity of C. lacerata was relatively high.
Conclusions
The results illustrated that formaldehyde inactivated Ceriporia lacerata biomass (without acid or alkali treatment for improving adsorption properties) showed a relatively high capacity in removal of copper(II) from aqueous solutions. The optimum operating conditions was proved to be at pH 6.0, contact time of 1 hour, initial copper(II) concentration of 200 mg/L. The pseudo second-order adsorptive model gave the best adjustment for copper(II) biosorption kinetics, while the equilibrium data fitted very well to both Langmuir and Freundlich adsorptive isotherm models. Without further acid or alkali treatment for improving adsorption properties, formaldehyde inactivated C. lacerata biomass possesses good biosorption characteristics on copper(II) removal from aqueous solutions. Prospectively, immobilized or further pretreated Ceriporia lacerata biomass has potential to be used as an efficient adsorbent in treatment of heavy metal polluted waters.
Acknowledgements
We are grateful for funding from the Key Project in the National Science and Technology Pillar Program during the Twelfth Five-year Plan Period (2011BAC09B01), the Key National Water Special Project of China (2012ZX07204004-003), the CAS Guiding Strategic Project for Science and Technology (XDA05050204), Guizhou R&D Program for Social Development (Qiankehe SY [2013]3144, Qiankehe SZ [2014]3036).
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
XL, AL and ML participated in the experimental activity, analysis and interpretation of the data; XT and XL participated in the design of the study and contributed to preparing the manuscript. All authors read and approved the final manuscript.
Contributor Information
Xiaona Li, Email: maidoulxn413@163.com.
Airong Li, Email: ai800102@gmail.com.
Mingzhong Long, Email: lmzlucky78@163.com.
Xingjun Tian, Email: tianxj@nju.edu.cn.
References
- 1.Sánchez A, Ballester A, Blázquez ML, González F, Muñoz J, Hammaini A. Biosorption of copper and zinc by Cymodocea nodosa. FEMS Microbiol Rev. 1999;23(5):527–36. doi: 10.1111/j.1574-6976.1999.tb00411.x. [DOI] [PubMed] [Google Scholar]
- 2.Çabuk A, Akar T, Tunali S, Gedikli S. Biosorption of Pb(II) by industrial strain of Saccharomyces cerevisiae immobilized on the biomatrix of cone biomas of Pinus nigra: equilibrium and mechanism analysis. Chem Eng J. 2007;131(1–3):293–300. doi: 10.1016/j.cej.2006.12.011. [DOI] [Google Scholar]
- 3.Pümpel T, Schinner F. Native fungal pellets as a biosorbent for heavy metals. FEMS Microbiol Rev. 1993;11(1–3):159–64. doi: 10.1016/0168-6445(93)90037-A. [DOI] [Google Scholar]
- 4.Ahluwalia SS, Goyal D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresource Technol. 2007;98(12):2243–57. doi: 10.1016/j.biortech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Kapoor A, Viraraghavan T. Fungal biosorption - an alternative treatment option for heavy metal bearing wastewaters: a review. Bioresource Technol. 1995;53(3):195–206. [Google Scholar]
- 6.Kapoor A, Viraraghavan T, Cullimore DR. Removal of heavy metals using the fungus Aspergillus niger. Bioresource Technol. 1999;70(1):95–104. doi: 10.1016/S0960-8524(98)00192-8. [DOI] [Google Scholar]
- 7.Say R, Denizl A, Arıca MY. Biosorption of cadmium(II), lead(II) and copper(II) with the filamentous fungus Phanerochaete chrysosporium. Bioresource Technol. 2001;76(1):67–70. doi: 10.1016/S0960-8524(00)00071-7. [DOI] [PubMed] [Google Scholar]
- 8.Treen-Sears M, Volesky B, Neufeld RJ. Ion exchange/complexation of the uranyl ion by Rhizopus biosorbent. Biotechnol Bioeng. 1984;26(11):809–14. doi: 10.1002/bit.260261109. [DOI] [PubMed] [Google Scholar]
- 9.Fourest E, Roux JC. Heavy metal biosorption by fungal mycelial by products: mechanisms and influence of pH. Appl Microbiol Biotechnol. 1992;37(3):399–403. doi: 10.1007/BF00211001. [DOI] [Google Scholar]
- 10.Veit MT, Tavares CRG, Gomes-da-Costa SM, Guedes TA. Adsorption isotherms of copper(II) for two species of dead fungi biomass. Process Biochem. 2005;40(10):3303–8. doi: 10.1016/j.procbio.2005.03.029. [DOI] [Google Scholar]
- 11.Tobin JM, Cooper DG, Neufeld RJ. Uptake of metal ions by Rhizopus arrhizus biomass. Appl Environ Microbiol. 1984;47(4):821–4. doi: 10.1128/aem.47.4.821-824.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Rome L, Gadd GM. Copper adsorption by Rhizopus arrhizus, Cladosporium resinae and Penicillium italicum. Appl Microbiol Biotechnol. 1987;26(1):84–90. doi: 10.1007/BF00282153. [DOI] [Google Scholar]
- 13.Özer A, Ekiz HI, Özer D, Kutsal T, Çağlar A. A stage purification process to remove heavy metal ions from wastewater using Rhizopus arrhizus. Process Biochem. 1991;32(44):319–26. [Google Scholar]
- 14.Bahadir T, Bakan G, Altas L, Buyukgungor H. The investigation of lead removal by biosorption: an application at storage battery industry wastewaters. Enzyme Microb Technol. 2007;41(1–2):98–102. doi: 10.1016/j.enzmictec.2006.12.007. [DOI] [Google Scholar]
- 15.Townsley CC, Ross IS. Copper uptake in Aspergillus niger during batch growth and in nongrowing mycelial suspension. Exp Mycol. 1986;10(4):281–8. doi: 10.1016/0147-5975(86)90013-7. [DOI] [Google Scholar]
- 16.Huang C, Huang CP, Morehart AL. Proton competition in Cu(II) adsorption by fungal mycelia. Water Res. 1991;25(11):1365–75. doi: 10.1016/0043-1354(91)90115-7. [DOI] [Google Scholar]
- 17.Rostami K, Joodaki MR. Some studies of cadmium adsorption using Aspergillus niger, Penicillium austurianum, employing an airlift fermenter. Chem Eng J. 2002;89(1–3):239–52. doi: 10.1016/S1385-8947(02)00131-6. [DOI] [Google Scholar]
- 18.Niu H, Xu XS, Wang JH. Removal of lead from aqueous solutions by Penicillium biomass. Biotechnol Bioeng. 1993;42(66):785–7. doi: 10.1002/bit.260420615. [DOI] [PubMed] [Google Scholar]
- 19.Volesky B, May-Phillips HA. Biosorption of heavy metals by Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 1995;42(5):797–806. doi: 10.1007/BF00171964. [DOI] [PubMed] [Google Scholar]
- 20.Baldrian P. Interactions of heavy metals with white-rot fungi. Enzyme Microb Technol. 2003;32(1):78–91. doi: 10.1016/S0141-0229(02)00245-4. [DOI] [Google Scholar]
- 21.Bayramoğlu G, Bektaş S, Arıca MY. Biosorption of heavy metal ions on immobilized white-rot fungus Trametes versicolor. J Hazard Mater. 2003;B101(3):285–300. doi: 10.1016/S0304-3894(03)00178-X. [DOI] [PubMed] [Google Scholar]
- 22.Suhara H, Maekawa N, Kaneko S, Hattori T, Sakai K, Kondo R. A new species, Ceriporia lacerata, isolated from white-rotted wood. Mycotaxon. 2003;86:335–47. [Google Scholar]
- 23.Suhara H, Daikouku C, Takata H, Suzuki S, Matsufuji Y, Sakai K, et al. Monitoring of white-rot fungus during bioremediation of polychlorinated dioxin-contaminated fly ash. Appl Microbiol Biotechnol. 2003;62(5–6):601–7. doi: 10.1007/s00253-003-1284-9. [DOI] [PubMed] [Google Scholar]
- 24.Kim G, Lim Y, Song Y, Kim J. Decay fungi from playground wood products in service using 28S rDNA sequence analysis. Holzforschung. 2005;59(44):459–66. [Google Scholar]
- 25.Cui B, Wei Y, Dai Y. Polypores from Zijin Mountain, Jiangsu province. Mycosystema. 2006;25(1):9–14. [Google Scholar]
- 26.Xu L, Zhu Y, He XB, Han GM, Tian XJ. Evaluation of a new fungus Ceriporia lacerate strain P2—its ability to decolorize Alizarin Red and Methyl Orange. World J Microbiol Biotechnol. 2008;24(12):3097–104. doi: 10.1007/s11274-008-9822-3. [DOI] [Google Scholar]
- 27.Chowdhary A, Agarwal K, Kathuria S, Singh PK, Roy P, Gaur SN, et al. Clinical significance of filamentous basidiomycetes illustrated by isolates of the novel opportunist Ceriporia lacerata from the human respiratory tract. J Clin Microbiol. 2013;51(2):585–90. doi: 10.1128/JCM.02943-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim JH, Park YK, Kim JE, Lee SP, Kim BC, Jang BC. Crude extract of Ceriporia lacerata has a protective effect on dexamethasone-induced cytotoxicity in INS-1 cells via the modulation of PI3K/PKB activity. Int J Mol Med. 2013;32(1):179–86. doi: 10.3892/ijmm.2013.1364. [DOI] [PubMed] [Google Scholar]
- 29.Singh SK, Doshi A, Pancholy A, Pathak R. Biodiversity in wood-decay macro-fungi associated with declining arid zone trees of India as revealed by nuclear rDNA analysis. Eur J Plant Pathol. 2013;136(2):373–82. doi: 10.1007/s10658-013-0172-0. [DOI] [Google Scholar]
- 30.Lee EJ, Lee SP. Novel bioconversion of sodium glutamate to γ-Amino butyric acid by co-culture of Lactobacillus plantarum K154 in Ceriporia lacerata culture broth. Food Sci Biotechnol. 2014;23(6):1997–2005. doi: 10.1007/s10068-014-0272-4. [DOI] [Google Scholar]
- 31.Kim MJ, Kim JJ, Kim GH. Metal tolerance and sorption potential of indigenous basidiomycetous fungi. In: Proceeding of Korean Timber Engineering Society; 2011. p. 88–89.
- 32.Ho YS, Wase DAJ, Foster CF. Kinetic studies of competitive heavy metal adsorption by sphagnum peat. Environ Technol. 1996;7(1):71–7. doi: 10.1080/09593331708616362. [DOI] [Google Scholar]
- 33.Benquella B, Benaissa H. Cadmium removal from aqueous solution by chitin: kinetic and equilibrium studies. Water Res. 2002;36(10):2463–74. doi: 10.1016/S0043-1354(01)00459-6. [DOI] [PubMed] [Google Scholar]
- 34.Pino GH, de Mesquita LMS, Torem ML, Pinto GAS. Biosorption of cadmium by green coconut shell powder. Miner Eng. 2006;19(5):380–7. doi: 10.1016/j.mineng.2005.12.003. [DOI] [Google Scholar]
- 35.Langmuir I. The adsorption of gases on plane surfaces of glass, mica and platinum. J Am Chem Soc. 1918;40(9):1361–403. doi: 10.1021/ja02242a004. [DOI] [Google Scholar]
- 36.Sağ Y, Kaya A, Kutsal T. The simultaneous biosorption of Cu(II) and Zn(II) on Rhizopus arrhizus: application of the adsorption models. Hydrometallurgy. 1998;50(3):297–314. doi: 10.1016/S0304-386X(98)00065-6. [DOI] [Google Scholar]
- 37.Aksu Z, Gönen F, Demircan Z. Biosorption of chromium(VI) ions by Mowital B30H resin immobilized activated sludge in a packed bed: comparison with granular activated carbon. Process Biochem. 2002;38(2):175–86. doi: 10.1016/S0032-9592(02)00053-5. [DOI] [Google Scholar]
- 38.Galli E, Mario FD, Rapanà P, Lorenzoli P, Angelini R. Copper biosorption by Auricularia polytricha. Lett Appl Microbiol. 2003;37(2):133–7. doi: 10.1046/j.1472-765X.2003.01354.x. [DOI] [PubMed] [Google Scholar]
- 39.Ozer A, Ozer E. Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto S. cerevisiae: determination of biosorption heats. J Hazard Mater. 2003;B100(1–3):219–29. doi: 10.1016/S0304-3894(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 40.Iqbal M, Edyvan RGJ. Biosorption of lead, copper and zinc ions on loofa sponge immobilized biomass of Phanerochaete chrysosporium. Miner Eng. 2004;17(2):217–23. doi: 10.1016/j.mineng.2003.08.014. [DOI] [Google Scholar]
- 41.Tewari N, Vasudevan P, Guha BK. Study on biosorption of Cr(VI) by Mucor hiemalis. Biochem Eng J. 2005;23(2):185–92. doi: 10.1016/j.bej.2005.01.011. [DOI] [Google Scholar]
- 42.Asmal M, Khan AH, Ahmad S, Ahmad A. Cole of sawdust in the removal of copper(II) from industrial wastes. Water Res. 1998;32(10):3085–91. doi: 10.1016/S0043-1354(98)00067-0. [DOI] [Google Scholar]
- 43.Huang CP, Ostovic FB. Removal of cadmium(II) by activated carbon adsorption. J Environ Eng. 1978;104(5):863–78. [Google Scholar]
- 44.Reed BE, Arunachalam S. Use of granular activated carbon columns for lead removal. J Environ Eng. 1994;120(2):416–36. doi: 10.1061/(ASCE)0733-9372(1994)120:2(416). [DOI] [Google Scholar]
- 45.Beolchini F, Pagnanelli F, Reverberi AP, Vegliò F. Copper biosorption onto Rhizopus oligosporus: pH-edge tests and related kinetic and equilibrium modeling. Int Eng Chem Res. 2003;42(20):4881–7. doi: 10.1021/ie020829h. [DOI] [Google Scholar]
- 46.Li XN, Xu QY, Han GM, Zhu WQ, Chen ZH, He XB, et al. Equilibrium and kinetic studies of copper(II) removal by three species of dead fungal biomasses. J Hazard Mater. 2009;165:469–74. doi: 10.1016/j.jhazmat.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 47.Nuhoglu Y, Oguz E. Removal of copper(II) from aqueous solutions by biosorption on the cone biomass of Thuja orientalis. Process Biochem. 2003;38:1627–31. doi: 10.1016/S0032-9592(03)00055-4. [DOI] [Google Scholar]
- 48.Gabriel J, Baldrian P, Hladíková K, Háková M. Copper sorption by native and modified pellets of wood-rotting basidiomycetes. Lett Appl Microbiol. 2001;32(3):194–8. doi: 10.1046/j.1472-765x.2001.00888.x. [DOI] [PubMed] [Google Scholar]
- 49.Yan C, Wang S, Zeng A, Jin X, Xu Q, Zhao J. Equilibrium and kinetics of copper(II) biosorption by Myriophyllum spicatum L. J Environ Sci. 2005;17(6):1025–9. [PubMed] [Google Scholar]
- 50.Taty-Costodes VC, Fauduet H, Porte C, Delacroix A. Removal of Cd(II) and Pb(II) ions from aqueous solutions by adsorption onto sawdust of Pinus sylvestris. J Hazard Mater. 2003;B105(1–3):121–42. doi: 10.1016/j.jhazmat.2003.07.009. [DOI] [PubMed] [Google Scholar]
- 51.Kapoor A. Removal of heavy metals from aqueous solution by Aspergillus niger. PhD Thesis. Regina: University of Regina;1992.
- 52.Volesky B. Biosorption and me. Water Res. 2007;41(18):4017–29. doi: 10.1016/j.watres.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 53.Akar T, Tunali S. Biosorption performance of Botrytis cinerea fungal by-products for removal of Cd(II) and Cu(II) ions from aqueous solutions. Miner Eng. 2005;18(11):1099–109. doi: 10.1016/j.mineng.2005.03.002. [DOI] [Google Scholar]
- 54.Han R, Li H, Li Y, Zhang J, Xiao H, Shi J. Biosorption of copper and lead ions by waste beer yeast. J Hazard Mater. 2006;B137(3):1569–76. doi: 10.1016/j.jhazmat.2006.04.045. [DOI] [PubMed] [Google Scholar]


