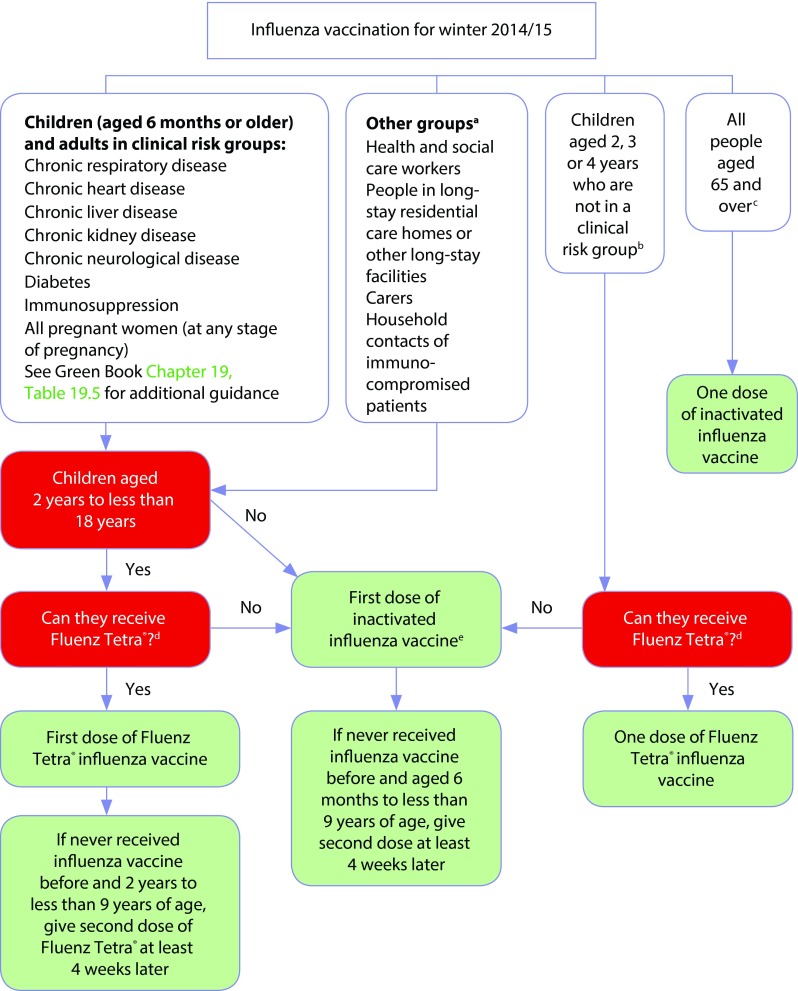
Figure 1.
Algorithm for influenza vaccination for winter 2014–2015 from Influenza: the Green Book [1].
aFollow additional guidance from UK health departments; ball children aged 2, 3, or 4 years (but not ≥5 years) on or before 1 September 2014*; call those aged ≥65 years, including all those aged 65 years on or before 1 March 2015; dif quadrivalent inactivated vaccine is available, consider for children aged ≥3 years only. If quadrivalent is not available, offer a suitable trivalent inactivated influenza vaccine. See Table 19.6 in Influenza: the Green Book [1], which lists the vaccines that can be used in young children: some are not suitable for young children; ecannot receive if: aged <2 years; aged ≥18 years; have egg allergy; have a history of active wheezing at the time of vaccination (until ≥7 days after wheezing has stopped); taking oral corticosteroids or high-dose inhaled corticosteroids for asthma; have certain immunodeficiencies; pregnant. This chart should be read in conjunction with the contraindications and precautions sections as well as Table 19.6 in Influenza: the Green Book [1], which gives details about the age indications for influenza vaccines.
*Note: In addition to these age groups, the administrations of constituent countries will also offer influenza vaccination to children aged 5 years (Scotland), all primary school children (Scotland and Northern Ireland) and children in year 7 (Wales).