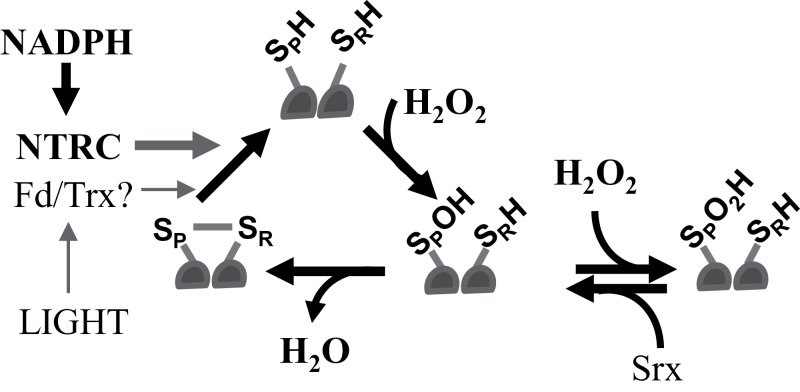
Fig. 8.
The control of the redox status of chloroplast 2-Cys Prxs. Typical chloroplast 2-Cys Prxs are homodimeric thiol peroxidases containing a peroxidatic (SPH) and resolving (SRH) cysteine residue in each monomer. The thiolic form of the peroxidatic cysteine attacks the peroxide and becomes transiently oxidized as sulfenic acid (-SPOH). This intermediate is then attacked by the resolving cysteine of the other subunit releasing a molecule of water and rendering both cysteines linked by a disulfide bridge. Alternatively, the sulfenic intermediate may be overoxidized to sulfinic acid (-SPO2H), which is retroreduced by Srx. Previous work has shown that NTRC, which uses NADPH as source of reducing power, is the most relevant reductant of the disulfide-bonded form of 2-Cys Prxs in Arabidopsis chloroplasts. In agreement with the latter, the ntrc-srx double mutant shows a phenotype similar to the ntrc mutant, indicating the relevance of NTRC for determining the level of 2-Cys Prxs overoxidation. Moreover, our results uncovered an NTRC-independent component contributing to 2-Cys Prx overoxidation. The fact that this component is light dependent suggests the participation of the Fd/Trx system.