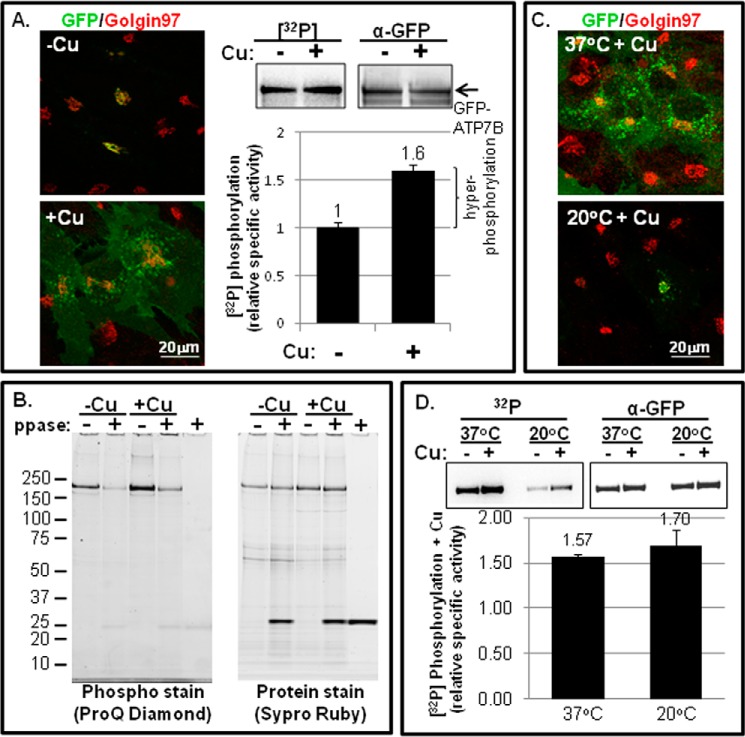
FIGURE 1.
Copper-stimulated ATP7B trafficking and Ser/Thr phosphorylation in fibroblasts. A, YS cells were incubated overnight in 25 μm TTM (−Cu) or overnight in basal medium followed by 1.5 h in 100–200 mm CuCl2 (+Cu). Cells were then fixed and labeled with antibodies to GFP (green) and Golgin 97 (left panels; single confocal images are shown), or they were metabolically labeled with [32P]orthophosphate followed by ATP7B immunoprecipitation and gel electrophoresis (right panels; representative autoradiogram of 32P incorporation and corresponding immunoblot of protein are shown). The bar graph (bottom right) shows the relative specific activities ±copper. The bracket indicates the amount of hyperphosphorylated ATP7B in the presence of copper relative to the basal phosphorylation observed in the presence of TTM (n = 13). B, phosphorylated GFP-WTATP7B was isolated from YS cells exposed to either 25 μm TTM (−Cu) or 100–200 μm copper (+Cu) and then treated with phosphatase or vehicle (ppase; + or −). Following immunoprecipitation and electrophoresis, phosphorylated GFP-ATP7B was detected using ProQ Diamond (left gel), and then the gel was reprobed for total protein using SYPRO Ruby stain (right gel). C, cells equilibrated at 37 (top panel) or 20 °C (bottom panel) were exposed to copper for 1.5 h, then fixed, and stained as described in A. D, YS cells were equilibrated at 37 or 20 °C and then metabolically labeled with [32P]orthophosphate followed by ATP7B immunoprecipitation as described in A. Gels on the left are autoradiograms; gels on the right are immunoblots for total ATP7B protein. The bar graph shows the specific activity (+Cu) normalized to chelator-treated cells (−Cu). The mean of three independent experiments is shown; error bars represent S.E.