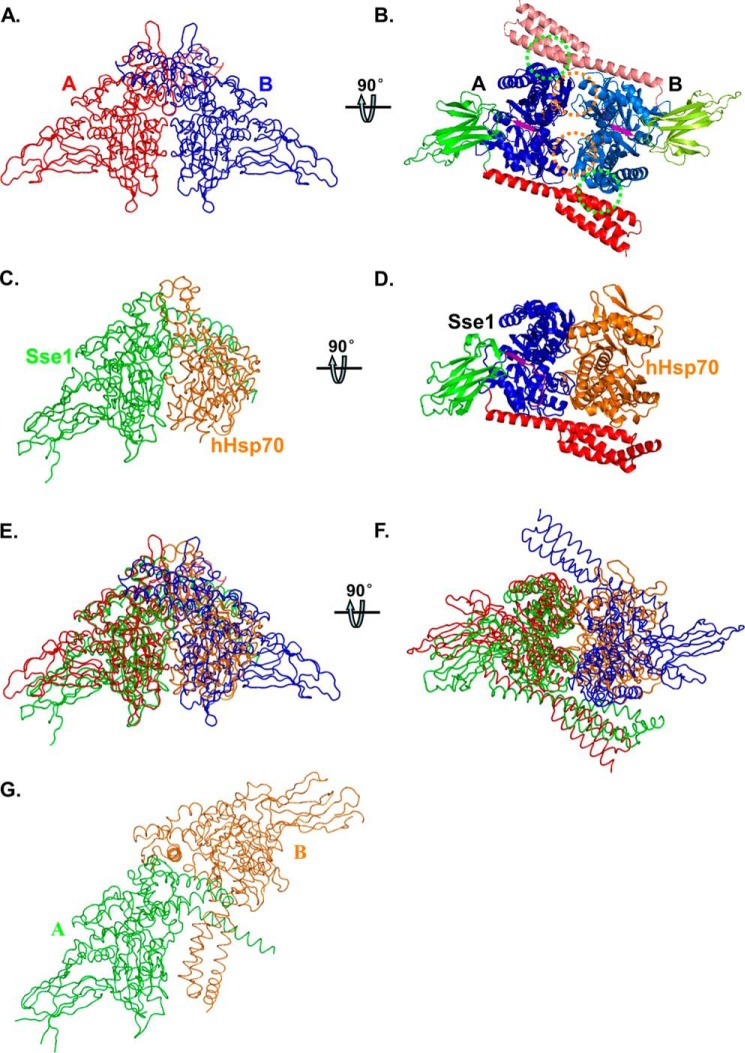
FIGURE 1.
Structure of the DnaK-ATP dimer. A and B, worm and ribbon diagrams of the DnaK-ATP homodimer, respectively. A, view has the 2-fold axis vertical and the NBD-NBD′ interface along the line of sight. The two DnaK protomers A and B are colored in red and blue in A, respectively. B, view is rotated 90° from A about a horizontal axis to view the DnaK dimer along the 2-fold axis from below. The DnaK protomers are colored based on domain as follows: NBD (blue for protomer A and marine for protomer B), inter-domain linker (purple), SBDβ (green for protomer A and lemon green for protomer B), and SBDα (red for protomer A and salmon for protomer B). NBD-NBD′ contacts are highlighted with orange circles, and the SBDa-NBD′ contacts are highlighted with green circles. C and D, worm and ribbon diagrams of the Sse1-hHsp70(NBD) heterodimer (Protein Data Bank code 3D2F), respectively. C, Sse1 is colored green and hHsp70(NBD) colored orange. The NBD of Sse1 is superimposed onto the NBD of DnaK protomer A in A, thereby providing an (A)-equivalent view of this complex. D, Sse1 is colored based on domain as in A, and hHsp70(NBD) is colored orange. The view is rotated relative to that in C just as B is rotated relative to A. E, superposition of A and C. F, superposition of B and D in worm diagrams. G, worm diagram of the Sse1 dimer (Protein Data Bank code 2QXL). The two protomers are colored in green and orange, respectively. The NBD of Sse1 protomer A is superimposed onto the NBD of DnaK protomer A in A, thereby providing an (A)-equivalent view of this complex.