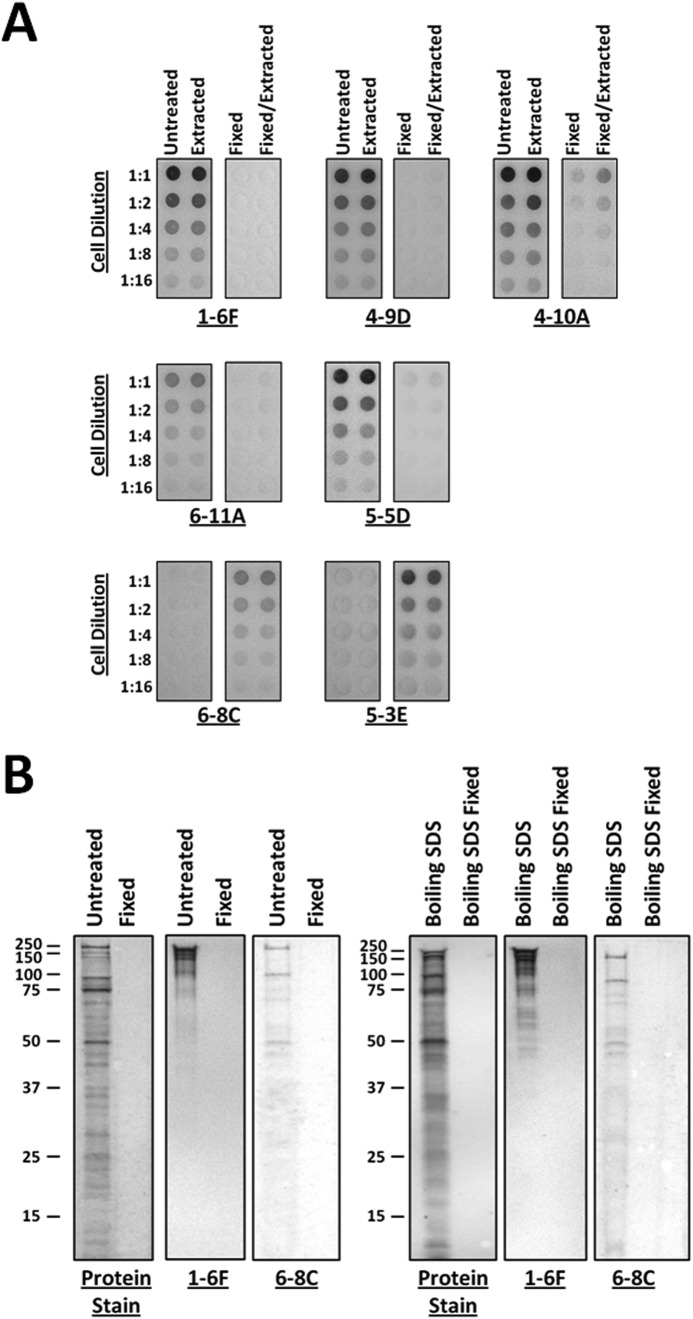
FIGURE 3.
Dot blots of unfixed and glutaraldehyde-fixed S. mutans and comparison of P1 fragments released from S. mutans by mechanical and SDS extraction. A, the degree of reactivity of a panel of different anti-P1 mAbs, which map to distinct regions of the P1 tertiary structure (49) (Fig. 1C), was evaluated against fixed and unfixed cells both before and after mechanical extraction. The overall antibody reactivity profile was essentially unaffected by mechanical extraction. However, antigenicity following fixation was notably altered. The epitopes recognized by mAbs 1-6F, 4-9D, 4-10A, 6-11A, and 5-5D were masked following fixation, whereas the C-terminal epitopes recognized by mAbs 6-8C and 5-3E became apparent (49). B, SDS-PAGE and Western blots of proteins released from untreated or glutaraldehyde-fixed cells by mechanical extraction or boiling in SDS. The profiles of non-covalently linked P1 fragments released by both methods appear to be similar. P1 fragments recognized by mAbs 1-6F and 6-8C, which map to the globular head and C-terminal segments of the molecule, respectively (49) (Fig. 1C), are present in both samples. No detectable P1 fragments were released from the fixed cells by either method.