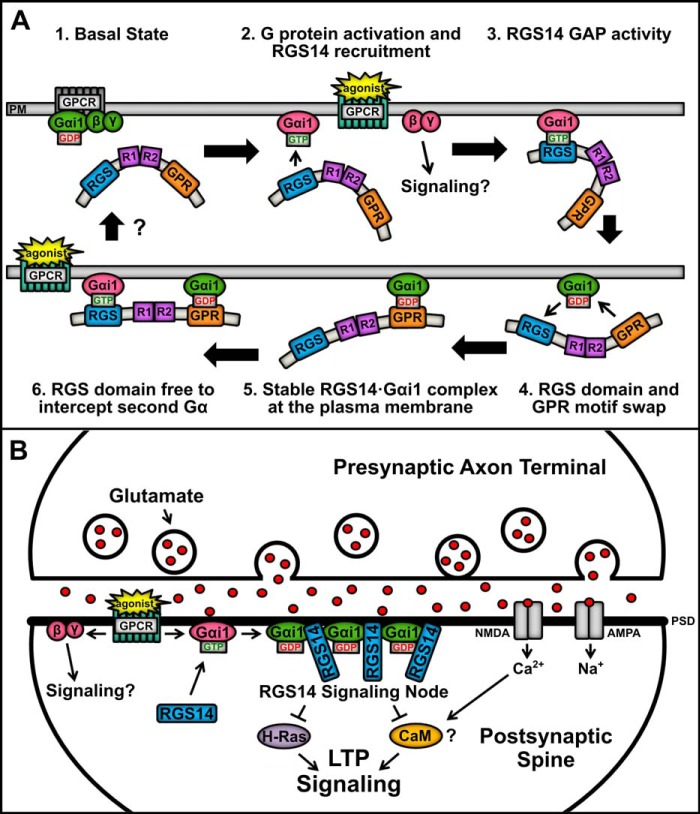
FIGURE 9.
Proposed model showing RGS14 binding and integration of Gα-GDP and Gα-GTP signaling in cells. A, the proposed model of RGS14 signaling function proceeds clockwise from the top left. (1) In the basal resting state, Gαi1-GDP in complex with Gβγ is bound to a GPCR at the plasma membrane (PM), whereas RGS14 remains in the cytosol. (2) Agonist activation of a GPCR induces downstream signaling through Gβγ, whereas activated Gαi1-GTP recruits RGS14 to the plasma membrane. (3) RGS14 accelerates the GTPase of Gαi1, causing hydrolysis of GTP to GDP. (4) The RGS domain loses affinity for Gαi1-GDP, and the GPR motif is free to bind the newly inactivated Gαi1. (5) RGS14 forms a stable complex with inactive Gαi1-GDP at the plasma membrane, which could serve to nucleate local recruitment of other RGS14·Gαi1-GDP complexes. (6) The RGS domain is then free to intercept and “GAP” a second near Gαi1-GTP after activation of a nearby GPCR, generating a Gαi1-GDP that could recruit a second RGS14. Unresolved (?) is how the complex is regulated to return to basal state (1). B, in the dendritic spines of CA2 hippocampal neurons, RGS14 is recruited to the PSD by activated Gαi1-GTP following coincident activation of a GPCR (e.g. metabotropic glutamate receptors and others) and iGluRs (AMPA and NMDA receptors). Following RGS domain-catalyzed GTP hydrolysis, RGS14 may remain bound at the membrane by inactive Gαi1-GDP through the GPR domain, thereby concentrating RGS14 at the PSD. This serves to nucleate subsequent recruitment of a collection of RGS14·Gαi1 complexes at the PSD to form a signaling node that can intercept incoming excitatory signals promoting LTP, such as H-Ras and Ca2+/calmodulin (CaM). Clustering of inactive Gαi1-GDP in an RGS14·Gαi1 signaling node may also promote sustained signaling by activated Gβγ.