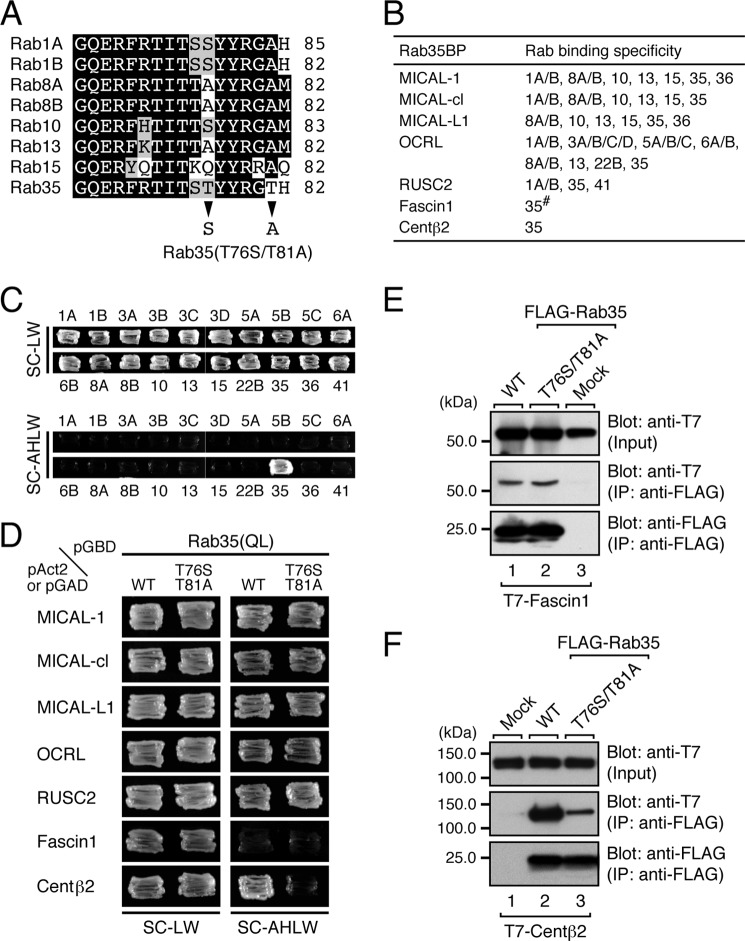
FIGURE 1.
Identification of critical residues responsible for the specific binding of centaurin-β2 in the switch II region of Rab35 by site-directed mutagenesis. A, sequence alignment of the switch II regions of mouse Rab1A/B, Rab8A/B, Rab10, Rab13, Rab15, and Rab35. Amino acid residues in the sequences that are conserved in more than four switch II regions and that are similar are shown against a black background and a shaded background, respectively. Only two amino acids (arrowheads) in the switch II region of Rab1A/B and Rab35 are different, and we replaced the Thr-76 and Thr-81 of Rab35 with Ser and Ala, respectively, by site-directed mutagenesis (i.e. produced a T76S/T81A mutant, which mimics the switch II region of Rab1A). B, summary of Rab35BPs and their Rab binding specificity. The Rab binding specificity of all of the Rab35BPs except Fascin1 (#) has already been thoroughly investigated by yeast two-hybrid assays (7, 8, 12). C, centaurin-β2 specifically recognized Rab35 but did not recognize other Rabs that interact with MICALs, OCRL, and RUSC2. Yeast cells containing pAct2-cenaurin-β2-ANKR and pGBD-C1-Rabs(QL)ΔCys (7) were streaked on SC-LW (top panels) and SC-AHLW (selection medium; bottom panels) and incubated at 30 °C for 1 day and 1 week, respectively. D, two Thr residues of Rab35, Thr-76 and Thr-81, are critical for binding centaurin-β2 but not for binding other Rab35BPs. Yeast cells containing the pAct2 (or pGAD) plasmid expressing Rab35BP and pGBD plasmid expressing the constitutively active form (Rab35(QL)) of Rab35(WT) or Rab35(T76S/T81A) mutant were streaked on SC-LW (left panels) and SC-AHLW (right panels) and incubated at 30 °C for 1 day and 1 week, respectively. Note that the Rab35 containing the T76S/T81A mutations specifically abrogated binding activity toward centaurin-β2 (bottom right panel). E, the T76S/T81A mutation did not impair Facsin1 binding activity in co-immunoprecipitation assays. T7-tagged Facsin1 and FLAG-tagged Rab35(WT) or Rab35(T76S/T81A) mutant were co-expressed in COS-7 cells, and their associations were analyzed by co-immunoprecipitation assays with anti-FLAG tag antibody-conjugated agarose beads as described previously (29, 31). Co-immunoprecipitated T7-tagged Facsin1 (middle panel) and immunoprecipitated FLAG-tagged Rab35(WT) or Rab35(T76S/T81A) mutant (bottom panel) were detected with HRP-conjugated anti-T7 tag antibody and HRP-conjugated anti-FLAG tag antibody, respectively. Input, of the volume of the reaction mixture used for immunoprecipitation (IP) (top panel). The positions of the molecular mass markers (in kilodaltons) are shown on the left. F, the T76S/T81A mutation dramatically decreased the centaurin-β2 binding activity of Rab35, a finding that was consistent with the results of the yeast two-hybrid assays shown in D. Co-immunoprecipitation assays were performed as described in E.