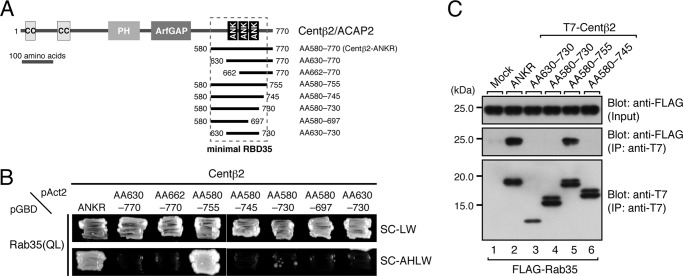
FIGURE 5.
Determination of the minimal Rab35-binding site in centaurin-β2. A, schematic representation of the deletion mutants of the ANKR domain of centaurin-β2 used in this study. AA numbers are shown on both sides of each construct. The minimal RBD35 determined in this study is indicated by enclosure with a broken line. B, Rab35 binding activities of the centaurin-β2 deletion mutants. Note that the ANKR domain of centaurin-β2 alone did not recognize Rab35 at all and that additional N-terminal and C-terminal regions adjacent to the ANKR domain were required for Rab35 binding activity. Yeast cells containing pAct2 plasmid expressing Centβ2-ANKR or each Centβ2-ANKR mutant (AA630–770, AA662–770, AA580–755, AA580–745, AA580–730, AA580–697, or AA630–730) and pGBD plasmid expressing Rab35(QL) were streaked on SC-LW (top panel) and SC-AHLW (bottom panel) and incubated at 30 °C. C, Rab35 binding activity of Centβ2-ANKR mutants (AA630–730, AA580–730, AA580–755, and AA580–745) in COS-7 cells. Co-immunoprecipitation assays were performed as described in the legend for Fig. 1E. The positions of the molecular mass markers (in kilodaltons) are shown on the left. IP, immunoprecipitation; PH, pleckstrin homology domain; ANK, ankyrin repeat.