FIGURE 1.
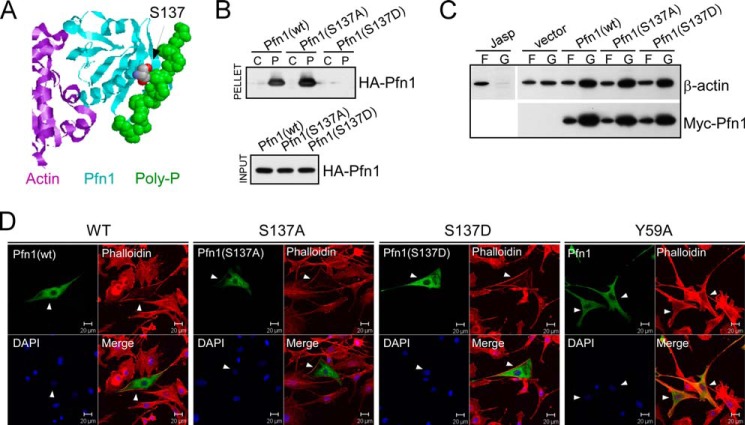
Mimicking Ser-137 phosphorylation selectively inhibits PLP binding of Pfn1. A, x-ray diffraction structure of human Pfn1 (middle, cyan) bound with actin (left, purple) and a 16-amino acid PLP peptide derived from the vasodilator-stimulated phosphoprotein (right, green, in space-filling mode) (Protein Data Bank ID 2PAV). Ser-137 of Pfn1, indicated by the arrow, is located next to the PLP peptide but far from actin. B, HEK293 cells were transfected with HA-tagged Pfn1 (wt, S137A, or S137D), and lysates were bound to control (C) or PLP-conjugated (P) Sepharose beads. Anti-HA antibody was used to detect HA-Pfn1 bound to the beads (pellet) and in the lysate (input) by Western blot. C, HEK293 cells transfected with pcDNA3 (vector) or Myc-tagged Pfn1 (wt, S137A, and S137D) were fractionated and analyzed for F-actin (F)/G-actin (G) ratio by Western blot. Jasplakinolide was used to stabilize F-actin before fractionation as a control. Similar expression of all three Myc-Pfn1 proteins was confirmed by Western blotting using a Myc-tag antibody. D, NIH 3T3 cells were transfected with Pfn1 constructs (wt, S137A, S137D, and Y59A) and subsequently co-stained for overexpressed Pfn1 with an anti-Pfn1 antibody and F-actin with rhodamine-phalloidin. Arrowheads point to positive cells which expressed the indicated Pfn1 plasmids.