FIGURE 1.
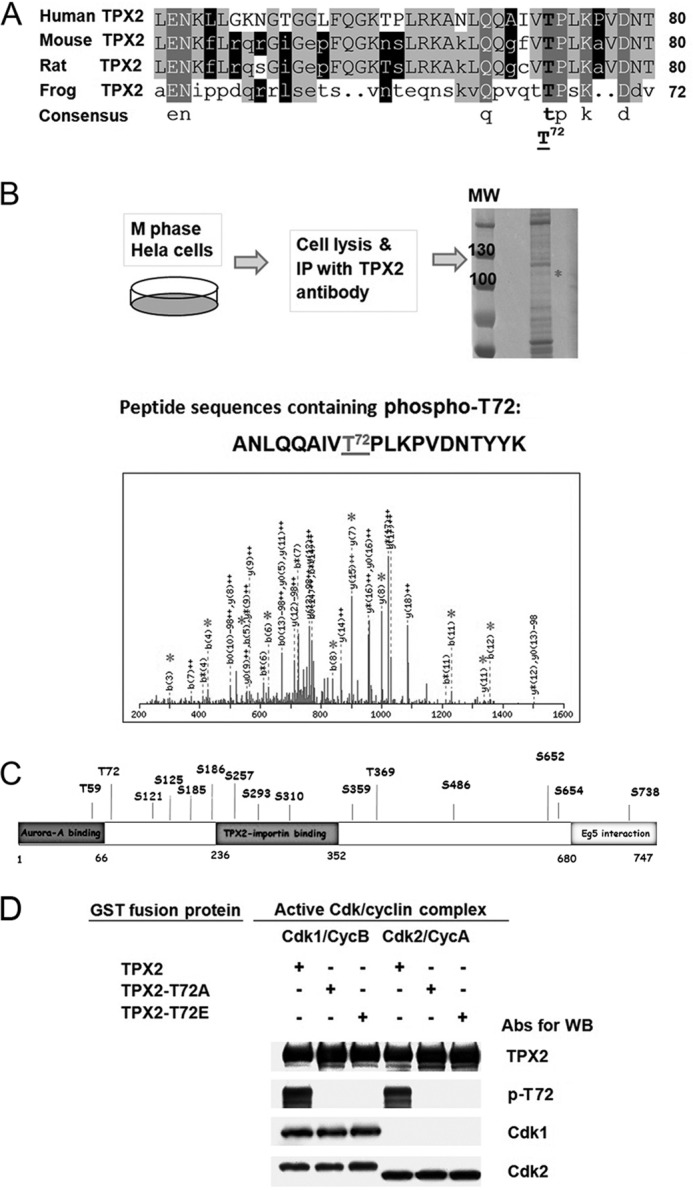
The evolutionary conserved Thr72 in human TPX2 is phosphorylated by Cdk1 and Cdk2 in vitro. A, comparative alignment of part of human TPX2 sequence (amino acids 41 to 80) with corresponding sequences from other species (mouse, rat, and frog) using DNAMAN software (Lynnon Corporation). The sequence alignment shows that Thr72 in human TPX2 is conserved in all other species. B, schematic diagram of the experimental protocol for mass spectrometry analysis (LC-MS/MS) using mitotic HeLa cells. HeLa cells were synchronized at M phase by nocodazole treatment (100 ng/ml) for 16 h and released for 30 min after nocodazole washout. Endogenous TPX2 was immunoprecipitated from 10 mg of total protein using pan-TPX2 Abs (clone 184). IP sample was run on SDS-PAGE and after Coomassie Blue staining, the band with the matching size to TPX2 (confirmed by Western blotting with TPX2 Abs, not shown) was cut out and sent for LC-MS/MS analysis. The gray asterisk on the spectra of the phosphopeptide containing Thr72 indicate the identified matched fragment ions on mass spectrometry. C, phosphorylation sites identified by mass spectrometry analysis on endogenous TPX2 immunoprecipitated from nocodazole-synchronized mitotic HeLa cells in regards to the known TPX2 domains. All these sites have been identified previously (17, 19–32) but not confirmed and analyzed. Thr72 is the first validated and functionally characterized phosphorylation site in human TPX2 (this study). D, in vitro kinase assay using purified Cdk1/2 proteins and phosphospecific Thr72 TPX2 Abs. Purified GST fusion protein TPX2 WT, GST-TPX2-T72A, or GST-TPX2-T72E was incubated with each active Cdk-cyclin complex in the presence of 1 mm cold ATP. All kinase reactions were stopped by adding 2× SDS sample buffer and the samples were run on SDS-polyacrylamide gel electrophoresis followed by Western blot detection using the indicated antibodies.
