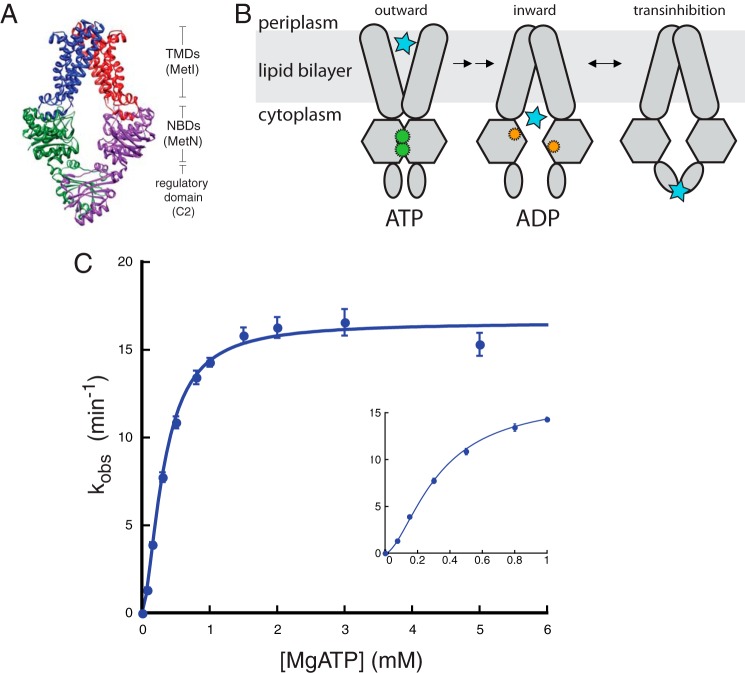
FIGURE 1.
Binding of ATP to MetNI is cooperative. A, representation of MetNI structure and domains within the cell membrane (11). B, alternating access model for transport with the addition of the transinhibition model. C, ATPase activity of MetNI in the absence of inhibitors. Inset, observed rate constant at low concentrations of MgATP. Reaction conditions consisted of 60 mm Tris, pH 7.5, 5 mm TAPS, pH 8.5, 0.055% DDM, 55 mm NaCl, 200 μm 2-amino-6-mercapto-7-methylpurine riboside, 0.1 units of purine nucleoside phosphorylase per 100 μl, and equimolar amounts of MgCl2 and ATP at 33 °C. Equations for fitting are described under “Experimental Procedures.” Error bars represent S.E. from at least three independent experiments.