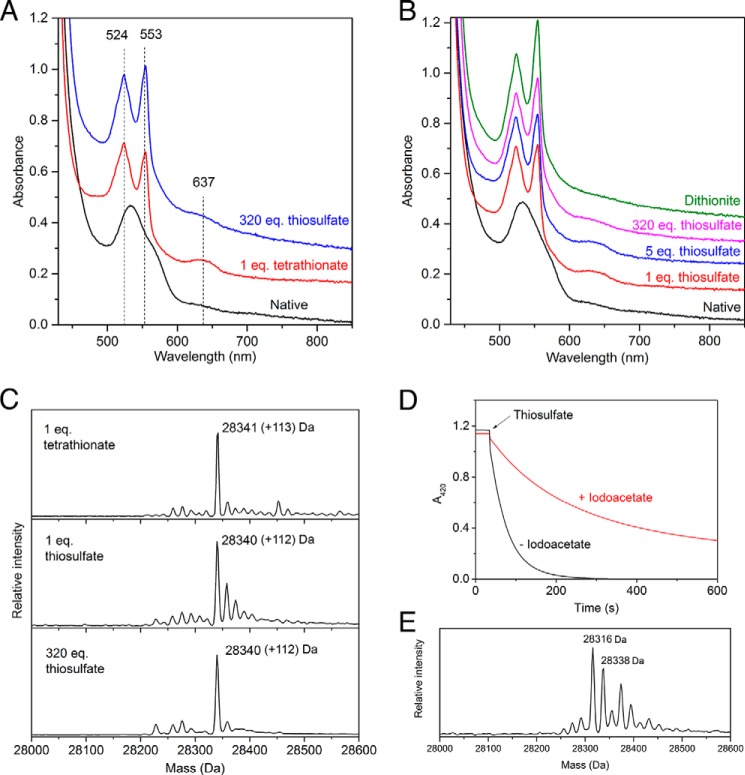
FIGURE 2.
Reactivity of TsdA with thiosulfate and tetrathionate at pH 4.25. A–C, 25 μm TsdA in 100 mm ammonium acetate, pH 4.25, was either left untreated (Native) or incubated for 3 min with the indicated equivalents of either thiosulfate or tetrathionate. A and B, visible spectra of a single sample to which the indicated additions were made successively in the order bottom to top. In B, dithionite was added until no further spectral changes were seen. For clarity, the spectra are offset relative to the native spectrum. C, samples were quenched in 0.1% TFA and subjected to ESI-MS. The expected mass increase for thiosulfonation is 112 Da. D, enzyme turnover removes thiosulfonation from Cys123. Reactions contained 1 nm S-thiosulfonated TsdA and 1 mm potassium ferricyanide in 100 mm ammonium acetate, pH 4.25, either in the presence or absence of 10 mm iodoacetate. The reaction was initiated by the addition of 8 mm thiosulfate, and oxidation of ferricyanide was monitored at 420 nm. E, the cysteine S-thiosulfonate group formed at the TsdA active site is unstable. Thiosulfonated TsdA was generated by the addition of tetrathionate at pH 8.0 and then desalted into 30 mm Tris-HCl, pH 8.0, 160 mm NaCl. This sample was incubated for 1 h at 37 °C in the presence of 20 mm sodium iodoacetate to capture any thiols formed, quenched in 0.1% TFA, and analyzed by ESI-MS. The expected mass of the starting cysteine S-thiosulfonate species is 28,340 Da, and the expected mass of a S-carboxymethylated cysteine persulfide adduct is 28,317 Da.