Abstract
Whether and how working memory disrupts or alters auditory selective attention is unclear. We compared simultaneous event-related potentials (ERP) and functional magnetic resonance imaging (fMRI) responses associated with task-irrelevant sounds across high and low working memory load in a dichotic-listening paradigm. Participants performed n-back tasks (1-back, 2-back) in one ear (Attend ear) while ignoring task-irrelevant speech sounds in the other ear (Ignore ear). The effects of working memory load on selective attention were observed at 130-210 msec, with higher load resulting in greater irrelevant syllable-related activation in localizer-defined regions in auditory cortex. The interaction between memory load and presence of irrelevant information revealed stronger activations primarily in frontal and parietal areas due to presence of irrelevant information in the higher memory load. Joint independent component analysis of ERP and fMRI data revealed that the ERP component in the N1 time-range is associated with activity in superior temporal gyrus and medial prefrontal cortex. These results demonstrate a dynamic relationship between working memory load and auditory selective attention, in agreement with the load model of attention and the idea of common neural resources for memory and attention.
Keywords: Attention, Auditory, Working memory load, fMRI, ERP, jICA
Introduction
In a selective attention task, sensory perceptual processing of task-irrelevant stimuli is determined by ongoing task characteristics. The level of difficulty on a goal directed task has long been thought to be a major factor in attentional selectivity (e.g., Sabri et al., 2013a). More recently, the load theory of selective attention proposed that the type of mental processing imposed by the task, perceptual versus cognitive control, is equally important (Lavie, 2010; Lavie et al., 2004). This model makes opposite predictions as to the effects of perceptual load and cognitive load on task-irrelevant information processing. Numerous studies, using behavioral and neuroimaging measures, have shown that indeed greater perceptual demand on a visual task is associated with reduced interference and successful selective attention, whereas higher demand on cognitive control (e.g., working memory, dual task) is associated with increased processing of irrelevant information (de Fockert, 2013; de Fockert et al., 2001; Kelley and Lavie, 2011; Rees et al., 1997; Schwartz et al., 2005; Xu et al., 2011). The effects of perceptual demand and working memory load on sensory processing of task-irrelevant visual distractors were observed as early as primary visual cortex (area V1) (Bahrami et al., 2007; Kelley and Lavie, 2011; Schwartz et al., 2005).
This dissociation between perception and cognitive control has been proposed as evidence in support of the view that there are two distinct mechanisms of attention. According to the load theory, a passive perceptual selection mechanism is engaged under situations when perceptual resources are exhausted (high perceptual load) whereas an active mechanism of cognitive control is involved in maintaining current task goals and reducing additional processing of irrelevant information. When a primary task requires cognitive control operations, maintenance of task goals are consequently compromised due to limited executive control resources (Lavie, 2005, 2010; Lavie et al., 2004). A functional magnetic resonance imaging (fMRI) study that compared the effects of both perceptual and working memory load on neural activity to irrelevant information reported that higher perceptual demand (degraded faces) on a face-repetition detection task reduced processing of task-irrelevant simultaneously presented scenes in the parahippocampal place area (Yi et al., 2004). In contrast to load theory predictions and previous reports, manipulation of memory load in Yi's study revealed similar levels of activation to the irrelevant scenes in both 0-back and 2-back tasks, suggesting a comparable competition between relevant and irrelevant information posed by the presented visual objects. An alternative explanation for the effects of load might involve hierarchical prediction processing models. In this context, differential activation in sensory cortex could be attributed to the computation of prediction errors, whereby a greater error is manifested as greater sensory processing of information that was not explained away by top-down predictions (Clark, 2013; Feldman and Friston, 2010; Friston and Kiebel, 2009; Garrido et al., 2007; Rao and Ballard, 1999).
Evidence on the neural effects of task demand and task type on processing irrelevant information in the auditory modality is sparse (cf. Alain and Izenberg, 2003). A recent fMRI study systematically manipulated load in a pitch discrimination and a pitch memory n-back task but focused on task effects in auditory cortex to attended relevant sounds (Rinne et al., 2009). We recently investigated the effects of perceptual load, modulated parametrically in a signal-detection task, on processing of task-irrelevant sounds using simultaneous recording of event-related potentials (ERPs) and fMRI. Consistent with findings in the visual modality, we found an inverse relationship between perceptual load and neural responses to irrelevant speech sounds, with a linear increase in the auditory cortex blood oxygen level dependent (BOLD) response and in the ERPs at 130-230 msec as perceptual demands decreased (Sabri et al., 2013b; cf. Sabri et al., 2006). In the present study, we seek to expand on the above results and examine the effects of cognitive control load on processing of task-irrelevant sounds, using an auditory n-back task. One ERP study that reported such a manipulation, using a sound sequence-matching task, demonstrated memory effects on attention in a dual-task paradigm in which inhibition of ignored standard sounds was delayed by approximately 50 msec from low to high memory load (Bidet-Caulet et al., 2010). Similar memory load manipulations using functional imaging techniques are lacking, and whether and how working memory disrupts or alters auditory selective attention is unclear.
Evidence on the relationship between selective attention and working memory comes from studies showing a replicable positive correlation on behavioral performance measures between the tasks (Kane and Engle 2003). In addition, fMRI research reveals an overlap between attention and working memory networks, including intraparietal sulcus (IPS), superior and middle frontal gyri (SFG, MFG), and frontal eye fields (FEFs), supporting the view that the two cognitive functions share neural resources (Awh et al., 2006; Corbetta et al., 2002; Cowan, 1998; Kastner and Ungerleider, 2000; Mayer et al., 2007; Zanto et al., 2011). The relationship between working memory performance and modulation of visual ERP components (P1, N1) by attention was demonstrated in a recent study, in which low performance trials were characterized by lack of neural suppression of irrelevant information (Zanto and Gazzaley, 2009).
The present study employed a pitch working memory task and a dichotic listening paradigm to investigate the relationship between working memory load and auditory attention, focusing primarily on sensory perceptual processing of task-irrelevant sounds in auditory cortex using simultaneous recordings of ERPs and fMRI. Our results show that higher working memory load is associated with greater neural processing of task-irrelevant sounds in auditory cortex in the N1 time range, demonstrating a negative correlation between memory task demand and selective attention. The results are in agreement with the load model of attention and the idea of common neural resources for memory and attention.
Material and Methods
Subjects
Participants were 20 healthy adults (10 men, mean age = 25 years, SD = 5) with no history of neurological or hearing impairments and normal or corrected-to-normal visual acuity. The participants were native English speakers, and all were right-handed according to the Edinburgh Handedness Inventory (Oldfield, 1971). Data from 7 subjects were excluded from ERP analysis (6 with noisy EEG, 1 due to equipment failure). Informed consent was obtained from each participant prior to the experiment, in accordance with the Medical College of Wisconsin Institutional Review Board.
Task Design and Procedure
The study employed a block design and a dichotic listening paradigm. There were eight simultaneous ERP/fMRI dichotic-listening runs, each divided into four blocks of 51 sec each. Each block was followed by a 12 sec rest period. A block was composed of seventeen 3 sec trials, in which a single 100 msec tone was presented to the Attend ear during the 1.2 sec between image acquisitions. Across the trials in each block, tones were 1000, 1790, or 3375-Hz with equal probability. An n-back memory task on the tones was modulated to create two memory load task conditions (NBACK1, NBACK2). The memory load was fixed within a block. Half of the blocks included task-irrelevant syllables presented to the Ignore ear. In these Syllable blocks, a single syllable (/ba/, /da/, /bi/, /di/, /bu/, /du/, /be/, /de/, /bo/, or /do/, selected randomly without replacement), 180 msec in duration, was presented to the Ignore ear on 10 of the trials. The tone (in the Attend ear) and syllable (in the Ignore ear) were presented such that they did not overlap temporally (ISI = 600 msec). Seven trials within the Syllable block did not include a syllable. Trials were pseudo-randomized within each block such that the ISI between successive syllables in the Syllable blocks was jittered exponentially between 3 and 15 sec. In the entire experiment, there were 80 syllable trials per task condition. Control blocks were identical except that speech sounds were not presented. Four blocks (2 Syllable and 2 Control for each of the NBACK1 and NBACK2 tasks) were delivered randomly within each run.
Participants performed an n-back matching task in the Attend ear and were instructed to ignore the irrelevant speech sounds presented to the other ear. The task condition, NBACK1 or NBACK2, was conveyed to the participants on the screen immediately before each block. Attend and Ignore ear designation was fixed within a run and varied pseudo-randomly across runs with equal assignment to each ear across the experiment. Participants were instructed to press button 1 for a match and button 2 for a mismatch. A cross-hair was presented in the middle of the screen to assist in minimizing eye movement.
An event-related localizer run, designed to identify areas sensitive to speech stimuli, followed the eight dichotic-listening runs. In the localizer run, participants discriminated between randomly presented 180 msec binaural tones and syllables by pressing buttons 1 and 2, respectively. The syllables were identical to those used in the dichotic-listening runs. Tones were ten logarithmically spaced sinewaves ranging from 200 to 4000 Hz. Stimulation consisted of 40 syllable and 40 tone events in randomized order, occurring during the 1.2 sec between image acquisitions. ISI was jittered exponentially between 3 and 9 sec.
The syllables were recorded from a male native English speaker and normalized according to loudness. Sounds were delivered through MRI-compatible STAX SR-003 electrostatic ear inserts (STAX, Saitama Prefecture, Japan), which were combined with a Bilsom over-the-ear muff providing approximately 23 dB of passive noise reduction (Bilsom, Sweden). The visual fixation stimulus was projected through an Epson LCD video projector onto an angled mirror located just above the eyes. Stimulus delivery was controlled by a personal computer running Presentation software (Neurobehavioral Systems, Inc. Albany, CA).
fMRI acquisition and analysis
Images were acquired on a 3T GE Excite scanner (GE Medical Systems, Milwaukee, WI). Functional data consisted of T2*-weighted, gradient-echo, echo-planar images (echo time = 25.7 msec, flip angle = 77°, acquisition time = 1.8 sec, delay = 1.2 sec), obtained using clustered acquisition at 3-sec intervals. Sounds were presented during the 1.2 sec period following each acquisition to avoid perceptual masking by the acoustic noise of the scanner. Functional images were composed of 33 axially-oriented 3 mm slices with a 0.5 mm interslice gap covering the whole brain, with FOV = 192 mm and 64 × 64 matrix, resulting in 3.0 × 3.0 × 3.5 voxel dimensions. High-resolution anatomical images of the entire brain were obtained using a 3-D spoiled gradient-echo sequence (SPGR) as a set of 130 contiguous axial slices with 0.938 × 0.938 × 1.0 mm voxel dimensions.
Image analysis was conducted using the AFNI software package (Cox, 1996). Within-subject analysis consisted of spatial registration to minimize motion artifacts (Cox and Jesmanowicz, 1999) and co-registration of functional and anatomy images (Saad et al., 2009). Voxel-wise multiple linear regression was applied to individual time series with reference functions, convolved with a gamma variate, separately representing the Syllable and Control blocks in NBACK1 and NBACK2 tasks. General linear tests were conducted between Syllable and Control blocks in each task condition to isolate the response to task-irrelevant syllables (e.g., Syllable_NBACK1 minus Control_NBACK1), as well as between task conditions to measure load effects. Individual coefficient maps were projected into standard stereotaxic space (Talairach and Tournoux, 1988) by linear re-sampling, and then smoothed with a Gaussian kernel of 6 mm FWHM. Group maps were created in a random-effects analysis. The group maps were thresholded at a voxel-wise p <.01 and corrected for multiple comparisons by removing clusters smaller than 898 μl, resulting in a map-wise two-tailed α=.05. This cluster threshold was determined through Monte-Carlo simulations that provide the chance probability of spatially contiguous voxels exceeding the voxel-wise p threshold.
Region of interest (ROI) analysis
The localizer run was analyzed in a similar fashion. The reference functions in the multiple regression represented the occurrence of a syllable or a tone. A general linear test between syllables and tones was conducted at the response peak to obtain regions sensitive to speech sounds. Group maps were created using a random-effects analysis. The group maps were thresholded at a voxel-wise p <.0005, and corrected for multiple comparisons by removing clusters smaller than 252 μl, resulting in a corrected map-wise two-tailed α=.05.
An ROI analysis was carried out within speech-sensitive areas in auditory cortex as defined by the contrast Syllables > Tones in the localizer. The average BOLD signal in the identified ROIs was extracted for the task-irrelevant syllables in each task condition, for each subject, and subjected to a paired t-test.
ERP acquisition and analysis
Sixty-four-channel EEG activity was acquired using the Maglink system (Neuroscan, Inc.) in a continuous mode, and the Quik-Cap electrode positioning system (Neuroscan, Inc.). Activity was recorded at full bandwidth and digitally sampled at 500 Hz per channel. Electrode sites conformed to the International 10-20 System with CPz serving as the reference. Vertical eye movements and electrocardiogram were each monitored with bipolar recordings. Inter-electrode resistance was kept below 5kΩ.
EEG analysis was conducted using the Scan 4.4 software package (Compumedics Neuroscan), focusing on task-irrelevant syllables. Initial within-subject analysis consisted of bandpass filtering at 0.1-30 Hz, ballistocardiogram artifact removal, creating epochs of –100 to +450 msec from each sound onset, baseline-correction of each epoch by removing the mean voltage value of the whole sweep, and rejection of epochs with voltage values exceeding +/−150 μv. The remaining epochs were then averaged according to each task condition. Each waveform was baseline corrected by subtracting the mean voltage of the pre-stimulus period from each point in the post stimulus interval. Grand-average waveforms were computed for task-irrelevant syllable events in the two task conditions. The resulting waveforms were digitally re-referenced to the mastoids. Mean amplitudes were extracted for each subject and averaged across fronto-central electrodes in the N1 time range (130-210 msec) in each condition and subjected to a paired t-test.
Joint ERP/fMRI data fusion
Averaged data from left and right frontal, central and posterior clusters were included in independent component (ICA) fusion analysis. Joint ICA allows for the combination of two modalities, ERP and fMRI, by jointly maximizing the spatial independence of the fMRI data and the temporal independence of the ERP data and identifying linked components that capture common inter-subject covariation (Calhoun et al., 2006). Spatial ICA of fMRI data was performed jointly with temporal ICA of ERP data across subjects deriving a spatiotemporal decomposition of fMRI and ERP components using the Infomax algorithm (Bell and Sejnowski, 1995), following the approach developed by Calhoun and colleagues (Calhoun et al., 2006). The spatial features were functional MR images representing the syllable-related responses in NBACK1 and NBACK2 task conditions for each subject. The temporal features were the ERPs time locked to the syllables in NBACK1 and NBACK2 for each subject.
Results
Behavioral performance
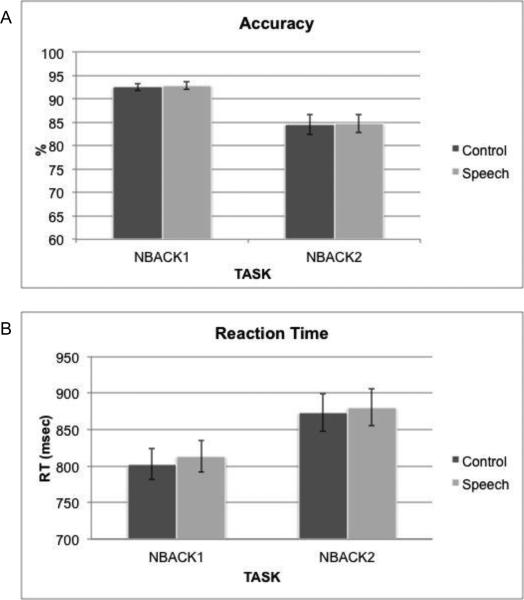
The mean accuracy performance measures for each combination of task (NBACK1, NBACK2) and irrelevant speech condition (Syllable, Control) are presented in Figure 1a. An ANOVA on the accuracy scores with Task and Irrelevant Speech as repeated measures revealed a main effect of Task [F(1, 19)=15.53, p<.001]. Accuracy was greater in NBACK1 than NBACK2, validating the main task manipulation. There was no main effect of Irrelevant Speech or any evidence for an interaction [F(1, 19)=.13 , p=.72; F(1, 19)=.01, p=.92].
Figure 1.
Mean accuracy (A) and RT (B) measures for the low and high working memory load tasks in the Control and Speech conditions. Error-bars indicate standard error (SEM).
The mean reaction time (RT) measures for each condition are presented in Figure 1b. An ANOVA on the RT scores with Task and Irrelevant Speech as repeated measures revealed a main effect of Task [F(1, 19)=15.49, p<.001]. Consistent with the accuracy results, RT was shorter in NBACK1 than in NBACK2. There was no main effect of Irrelevant Speech and no evidence for an interaction [F(1, 19)=2.04 , p=.17; F(1, 19)=.13, p=.72].
FMRI
Whole-brain analyses
Below we report only statistically significant effects (threshold z >2.5758, cluster correction α= .05, 898 μl). Table 1 provides the locations of local extrema for each contrast.
Table 1.
Locations of local extrema
| Contrast | x | y | z | Z-score | Anatomical Location |
|---|---|---|---|---|---|
| NBACK2 > NBACK1 | 37 | −49 | 41 | 5.691 | R SPL/IPS |
| −6 | 16 | 44 | 5.641 | L SFG | |
| −28 | 21 | 3 | 5.382 | L Insula | |
| 64 | −40 | −9 | 5.360 | R MTG | |
| −24 | −60 | 34 | 5.345 | L SPL/IPS | |
| −29 | 53 | 17 | 5.033 | L MFG/SFG | |
| 30 | −68 | 44 | 4.966 | R SPL | |
| −11 | −70 | 46 | 4.816 | L SPL/Precuneus | |
| −44 | −42 | 42 | 4.800 | L SPL | |
| −39 | 11 | 30 | 4.798 | L Inferior Frontal Sulcus/MFG | |
| 8 | −69 | 39 | 4.727 | R Precuneus | |
| 46 | 15 | 39 | 4.676 | R MFG | |
| 22 | 4 | 57 | 4.356 | R MFG | |
| 37 | 39 | 24 | 4.340 | R MFG | |
| −32 | −68 | 52 | 4.111 | L SPL | |
| 29 | −66 | 40 | 4.050 | R SPL | |
| −34 | 47 | 17 | 3.006 | L MFG | |
| NBACK1 > NBACK2 | −14 | 43 | 4 | −5.974 | L Anterior Cingulate |
| −9 | −39 | 44 | −5.867 | L Posterior Cingulate | |
| 30 | 3 | 11 | −5.654 | R Insula | |
| 9 | −22 | 41 | −5.446 | R Middle Cingulate | |
| 36 | −6 | −9 | −5.390 | R Insula | |
| 45 | −13 | 19 | −5.353 | R Insula | |
| −31 | −18 | 6 | −5.350 | L Insula | |
| −12 | 50 | 27 | −5.280 | L SFG | |
| 0 | 16 | 0 | −5.159 | L/R Anterior Cingulate | |
| 17 | −56 | 13 | −5.130 | R Parieto-Occipital Fissure | |
| −50 | 0 | −12 | −4.992 | L Anterior STG/STS | |
| −12 | 43 | 35 | −4.833 | L SFG | |
| 3 | 45 | 15 | −4.825 | R SFG/Anterior Cingulate | |
| 39 | 2 | −29 | −4.793 | R MTG/ITG | |
| −30 | 2 | 15 | −4.687 | L Insula | |
| 16 | −83 | 24 | −4.635 | R Cuneus | |
| 35 | −45 | −8 | −4.590 | R Fusiform Gyrus | |
| −29 | 32 | −6 | −4.565 | L OFC | |
| −20 | −14 | −11 | −4.489 | L Parahippocampal Gyrus | |
| 27 | −38 | 54 | −4.363 | R Postcentral Gyrus | |
| 38 | −32 | 17 | −4.349 | R Parietal Operculum | |
| −50 | −61 | 11 | −4.325 | L Middle Occipital Gyrus | |
| −3 | −67 | 20 | −4.300 | L Precuneus/Cuneus | |
| 60 | −38 | 31 | −4.055 | R SMG | |
| −53 | −2 | 8 | −4.037 | L IFG/Inferior Precentral Gyrus | |
| 40 | −72 | −5 | −3.991 | R Inferior Occipital Gyrus | |
| 19 | 11 | −17 | −3.789 | R Posterior Orbital Gyrus | |
| −33 | −52 | 21 | −3.789 | L Angular Gyrus | |
| 19 | −68 | −11 | −3.758 | R Fusiform Gyrus | |
| 14 | 30 | 50 | −3.720 | R SFG | |
| −33 | −81 | 11 | - | L Middle Occpital Gyrus | |
| 6 | 45 | 4 | 3.405 | R OFC | |
| −3.333 | |||||
| 5 | 16 | 27 | −3.225 | R Anterior Cingulate | |
| −8 | 22 | 58 | −3.207 | L SFG | |
| 33 | −17 | 36 | - | R Precentral Gyrus | |
| 41 | 6 | −26 | 3.137 | R MTG | |
| −3.073 | |||||
| Speech > Control | −54 | −18 | 11 | 5.998 | L STG |
| 49 | −29 | 8 | 5.711 | R Posterior STG | |
| 58 | −3 | 7 | 5.417 | R Anterior STG | |
| −42 | −39 | 12 | 5.195 | L Posterior STG | |
| −49 | 2 | 0 | 5.183 | L Anterior STG/Temporal Pole | |
| 7 | −43 | 33 | 4.672 | R Middle Cingulate | |
| 11 | −71 | 20 | 4.434 | R Cuneus | |
| −23 | −71 | 20 | 4.425 | L Superior Occipital Gyrus | |
| −18 | −58 | 3 | 4.339 | L Calcarine Gyrus | |
| −25 | −38 | −8 | 4.074 | L Fusiform Gyrus | |
| 57 | −46 | 18 | 3.933 | R Posterior STG | |
| 53 | 14 | 30 | 3.717 | R IFG | |
| 19 | −49 | 49 | 3.526 | R SPL | |
| −7 | −43 | 12 | 3.365 | L Posterior Cingulate | |
| −52 | −3 | 2 | 3.322 | L Anterior STG | |
| −56 | −12 | −14 | 3.200 | L MTG | |
| 37 | 2 | 29 | 3.164 | R Precentral Sulcus | |
| −46 | −28 | 5 | 2.929 | L Middle STG/STS | |
| S NBACK1 > C_NBACK1 | −35 | −31 | 5 | 4.788 | L STG/TTG |
| 50 | −30 | 4 | 4.653 | R STS | |
| 58 | −4 | −2 | 4.179 | R Anterior STG | |
| −55 | −11 | 4 | 4.158 | L Middle STG | |
| 22 | −20 | 1 | 3.100 | R Thalamus | |
| C_NBACK1 > S_NBACK1 | 33 | −29 | 57 | −3.910 | R Precentral Gyrus |
| S_NBACK2 > C_NBACK2 | 51 | −27 | 11 | 5.753 | R Posterior STG |
| −52 | −33 | 6 | 5.400 | L MTG | |
| −51 | −7 | −3 | 5.371 | L Anterior STG | |
| 39 | −16 | −7 | 5.058 | R Hippocampus/Insula | |
| 25 | 42 | 36 | 4.951 | R MFG | |
| −2 | 36 | 27 | 4.854 | L Anterior Cingulate Sulcus/SFG | |
| 58 | −51 | 3 | 4.850 | R MTG | |
| 5 | −59 | −1 | 4.758 | R Lingual Gyrus | |
| −8 | −67 | 14 | 4.708 | L Cuneus | |
| 16 | −73 | 19 | 4.669 | R Cuneus | |
| −3 | 10 | −7 | 4.646 | L Gyrus Rectus | |
| 53 | 4 | −4 | 4.630 | R Anterior STG | |
| 16 | −38 | −1 | 4.629 | R Lingual Gyrus/Parahippocampal Gyrus | |
| −63 | −48 | 17 | 4.480 | L Posterior STG | |
| −39 | 22 | 3 | 4.393 | L IFG | |
| 25 | −87 | 1 | 4.390 | R Middle Occipital Gyrus | |
| −13 | −46 | 45 | 4.374 | L Precuneus | |
| 49 | 16 | 29 | 4.298 | R IFG | |
| 8 | −53 | 39 | 4.277 | R Precuneus | |
| −21 | 52 | 30 | 4.218 | L SFG | |
| −9 | −81 | −6 | 4.125 | L Lingual Gyrus | |
| 13 | 26 | −2 | 4.094 | R Anterior Cingulate | |
| −3 | −3 | 42 | 4.091 | L Middle Cingulate | |
| −34 | −51 | −17 | 4.091 | L Fusiform Gyrus | |
| 36 | −60 | 9 | 4.078 | R Middle Occipital Gyrus | |
| 36 | −13 | 43 | 4.010 | R Precentral Gyrus | |
| 8 | −29 | 42 | 3.994 | R Middle Cingulate | |
| 28 | 7 | 30 | 3.938 | R Inferior Frontal Sulcus | |
| −9 | −39 | 4 | 3.937 | L Precuneus/Posterior Cingulate | |
| −31 | −80 | 8 | 3.920 | L Middle Occipital Gyrus | |
| −37 | −13 | 48 | 3.788 | L Precentral Gyrus | |
| 6 | −19 | −2 | 3.670 | R Thalamus | |
| 42 | 23 | −10 | 3.620 | R OFC | |
| 47 | −37 | 5 | 3.592 | R Posterior STS | |
| 40 | −38 | 46 | 3.532 | R SPL/Postcentral Gyrus | |
| 21 | −48 | 56 | 3.487 | R Postcentral Gyrus/SPL | |
| −66 | −29 | −9 | 3.476 | L MTG | |
| 14 | 5 | 57 | 3.368 | R SMA/SFG | |
| 12 | 8 | 8 | 3.118 | R Caudate | |
| −47 | −35 | 16 | 3.108 | L Posterior STG | |
| −32 | −16 | −24 | 3.090 | L Fusiform Gyrus | |
| −47 | −69 | −8 | 2.997 | L Inferior Occipital Gyrus | |
| 21 | 35 | 44 | 2.688 | R SFG | |
| 0 | −57 | 14 | 2.683 | L/R Precuneus | |
| Memory Load × Irrelevant Speech | 7 | −59 | −1 | 4.663 | R Lingual Gyrus |
| 64 | −21 | 19 | 4.585 | R SMG/Postcentral Gyrus | |
| 42 | −15 | 54 | 4.537 | R Precentral Gyrus | |
| 4 | 41 | 33 | 4.446 | R SFG | |
| 4 | −72 | 17 | 4.200 | R Cuneus | |
| 7 | 4 | 10 | 4.178 | R Caudate | |
| 3 | −6 | 43 | 4.165 | R Middle Cingulate/SMA | |
| 28 | −44 | 64 | 4.160 | R Postcentral Gyrus | |
| −35 | −44 | −15 | 4.061 | L Fusiform Gyrus | |
| −43 | −8 | −11 | 4.030 | L STS | |
| −47 | −6 | −37 | 3.986 | L ITG | |
| 25 | 47 | 35 | 3.946 | R MFG/SFG | |
| −12 | −90 | 27 | 3.806 | L Superior Occipital Gyrus | |
| 47 | −16 | 6 | 3.801 | R STG/TTG | |
| 5 | −26 | 70 | 3.661 | R SFG | |
| 42 | −16 | −28 | 3.660 | R ITG | |
| −19 | −60 | −6 | 3.632 | L Lingual Gyrus | |
| 19 | 27 | 42 | 3.574 | R SFG | |
| −21 | 23 | 43 | 3.560 | L MFG/SFG | |
| −17 | 46 | 34 | 3.549 | L MFG/SFG | |
| 12 | −94 | 19 | 3.528 | R Superior Occipital Gyrus | |
| 6 | −16 | −7 | 3.458 | R Thalamus | |
| −52 | −66 | 5 | 3.178 | L MTG | |
| −41 | 21 | 3 | 3.138 | L IFG |
Abbreviations: R=right; L=left; IFG=inferior frontal gyrus; IPS=mtraparietal sulcus; ITG=inferior temporal gyrus; MFG=middle frontal gyrus; MTG=middle temporal gyrus; OFC=orbital frontal cortex; SFG=superior frontal gyrus; SMA=supplementary motor area; SMG=supramarginal gyrus; SPL=superior parietal lobule; STG=superior temporal gyrus; STS=superior temporal sulcus; TTG=transverse temporal gyrus.
Memory load effect
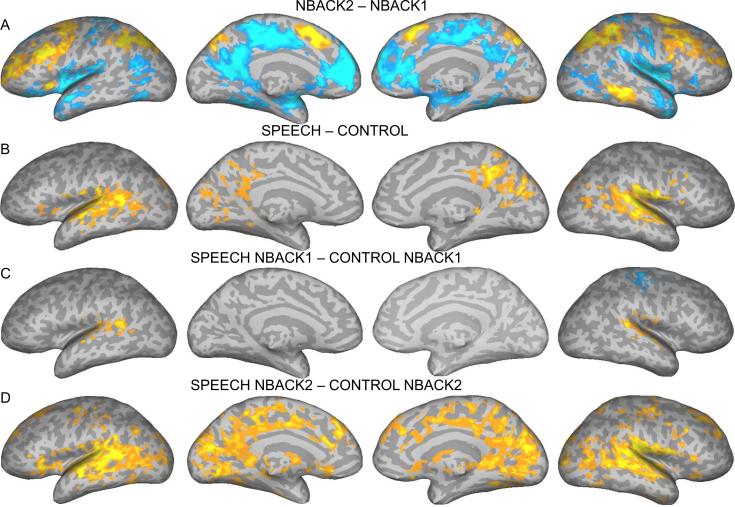
Stronger bilateral BOLD responses were seen in the NBACK2 condition compared to the NBACK1 condition (hot colors in Figure 2a) in brain areas associated with attention/working memory networks, including IPS, MFG (extending into the inferior frontal sulcus and precentral sulcus), posterior medial SFG (supplementary motor area, SMA), and FEF. Additional activation was observed in middle temporal gyrus (MTG). Higher BOLD signals for NBACK1 compared to NBACK2 were seen in the default mode network (DMN), including medial prefrontal cortex, rostral cingulate gyrus, posterior cingulate/precuneus, anterior temporal lobe, and fusiform gyrus bilaterally, and left angular gyrus (Figure 2a, in blue). Other regions more strongly activated in NBACK1 included the posterior insula, mid-cingulate gyrus, middle occipital gyrus, and central and postcentral gyri bilaterally. These same areas showed deactivation in the NBACK1 and NBACK2 compared to the resting baseline, suggesting that the higher activation levels observed for NBACK1 over NBACK2 were due to greater deactivation relative to resting in the higher demand NBACK2 condition.
Figure 2.
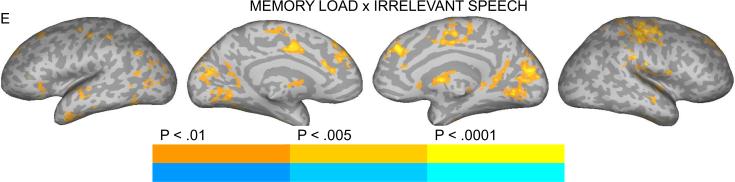
Brain activation for the contrast between NBACK2 and NBACK1 (main effect of task) (A), Speech and Control (main effect of irrelevant speech) (B), Speech and Control in the NBACK1 task (C), Speech and Control in the NBACK2 task (D), Interaction between Memory Load and Irrelevant Speech (E). The color scale indicates voxel-wise probability values.
Irrelevant Information effect
The contrast Syllable > Control revealed activation by the irrelevant speech syllables in anterior, middle, and posterior superior temporal gyrus (STG) bilaterally; posterior cingulate gyrus, precuneus, and cuneus bilaterally; inferior frontal gyrus (IFG) bilaterally; and left insula, fusiform gyrus, and lingual gyrus (Figure 2b).
Effect of memory load on irrelevant information processing
To examine the effects of cognitive control load on the processing of irrelevant information, the Syllable blocks for a given working memory load were contrasted with the corresponding Control blocks (Syllable_NBACK1 minus Control_NBACK1, hereafter S-C_NBACK1; Syllable_NBACK2 minus Control_NBACK2 , hereafter S-C_NBACK2).
The contrast S-C_NBACK1 revealed greater activity in bilateral STG (Figure 2c). The contrast S-C_NBACK2 revealed greater activity in anterior, middle, and posterior STG, MTG, and precentral gyrus bilaterally; medial prefrontal cortex, SMA, cuneus and lingual gyrus; left IFG and caudate; right SFG; and anterior cingulate sulcus and gyrus (Figure 2d).
The interaction between Memory Load and Irrelevant Speech (i.e., presence/absence of irrelevant information) (S-C_NBACK2 over S-C_NBACK1) is presented in Figure 2e. Inspection of the BOLD response profiles in regions, including STG/STS, SFG, right precentral/postcentral gyri, medial SFG, mid-cingulate gyrus, left angular gyrus, lingual gyrus and cuneus, revealed that the main phenomenon producing the interaction effects was stronger activation for Syllable_NBACK2 than Control_NBACK2 and the reverse under NBACK1 (stronger activation for Control_NBACK1 than Syllable_NBACK1).
ROI analyses
Localizer: Syllables > Tones
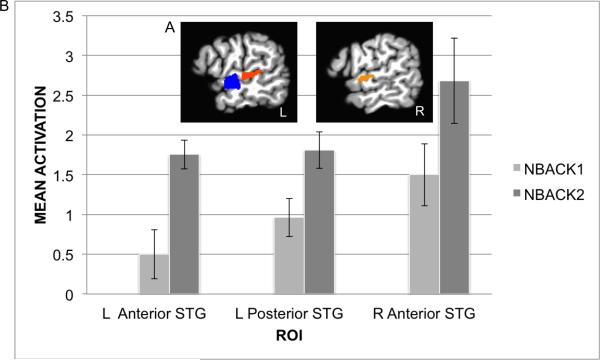
To identify neural regions related to speech processing, we contrasted Syllable and Tone activation in the localizer run. The contrast Syllables over Tones is presented in Figure 3a. Greater activation for syllables over tones was observed in three clusters: the anterior and middle-posterior portions of the left STG/STS [maxima = -57 -5 -8, -53 -20 1], and the anterior right STG [66 -20 -3] (threshold z>3.4809, cluster-corrected = .05, 252 Pl). The three identified clusters served as ROIs in the subsequent analysis.
Figure 3.
The syllable-sensitive ROIs as identified in the contrast Syllables>Tones in the localizer run (A). fMRI activation in the syllable-sensitive ROIs by irrelevant speech as a function of working memory load (B). Error-bars indicate standard error (SEM).
Effect of memory load on irrelevant information processing in auditory cortex
The difference between Syllable and Control in the left and right STG ROIs, as a function of working memory load (S-C_NBACK1, S-C_NBACK2), is depicted in Figure 3b. Mean activation was stronger in NBACK2, where working memory load is higher, than in NBACK1 in left anterior-middle STG [t(19)=3.43 , p<.005] and left posterior STG [t(19)=2.68 , p<.05], with a trend in right anterior-middle STG [t(19)=1.77, p=.09 n.s.].
Event-Related Potentials
Effect of memory load on irrelevant information processing
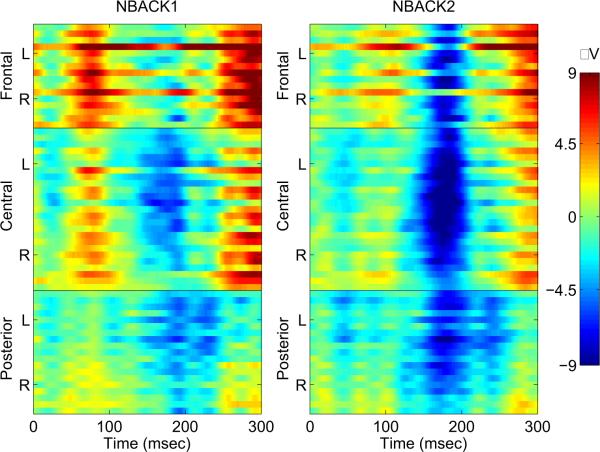
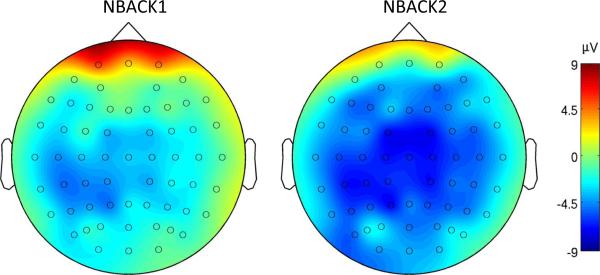
A negative deflection was observed in the N1 time window in the ERP response to task-irrelevant syllables in both task conditions (Figure 4, 5). Differences across load conditions for irrelevant syllables were observed approximately 130-210 msec after stimulus onset (Figure 6). This effect was quantified by computing the mean amplitude in this time range across fronto-central electrode sites, as a function of working memory load. The mean negativity was significantly higher in amplitude in NBACK2 compared to NBACK1 [t(12)=2.36, p<.05].
Figure 4.
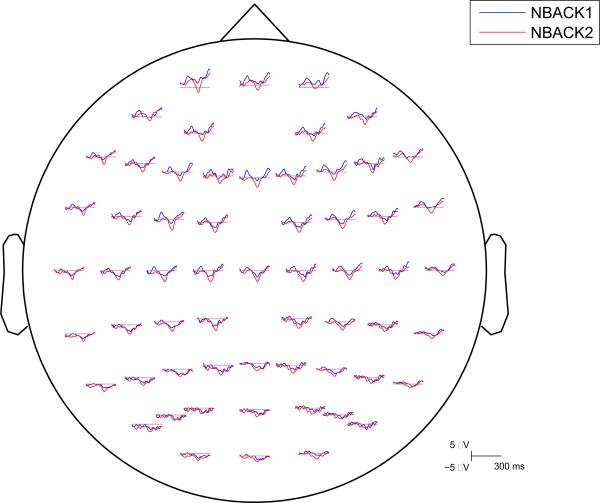
Spatio-temporal maps from 60 electrodes: Grand average ERPs of irrelevant syllables at each electrode as a function of memory load. The y-axis represents the frontal, central, and posterior electrodes. Each group of electrodes (frontal, central, posterior) is arranged top to bottom according to their lateral position from left (L) to right (R) with the midline electrode in the middle. The color scale represents the amplitude in PV.
Figure 5.
Group average ERP waveforms superimposed for irrelevant syllables in NBACK1 and NBACK2. Electrode sites conformed to the International 10-20 System.
Figure 6.
Mean scalp distribution for irrelevant syllables in the 130-210 msec time window, in NBACK1 and NBACK2. The color scale represents the amplitude in PV.
Joint ERP/fMRI
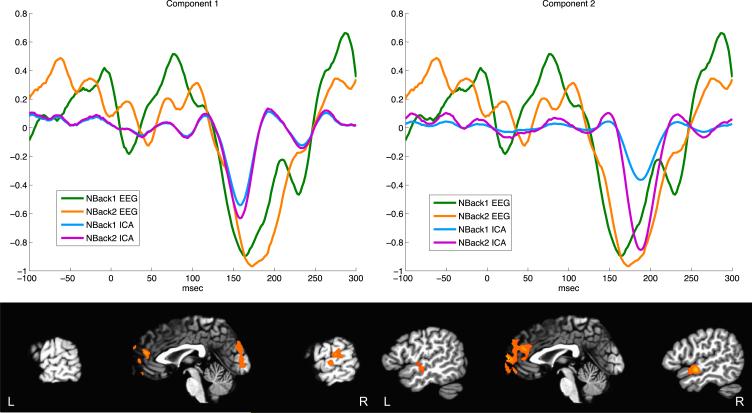
Using jICA on the fMRI and ERP data simultaneously allowed identification of brain regions that covary with specific time courses in the ERP data. There were two jICAERP components associated with the N1 time-range. In the fMRI maps these jICA components activated primarily in STG [maxima: Comp 1 = 58 -22 15; Comp 2 = 52 -5 -6, -59 -6 7] and medial prefrontal cortex [Comp 1 = -17 54 1, -6 62 17; Comp 2 = 14 59 11]. The jICA-fMRI maps and estimated jICA-ERPs are plotted together in Figure 7.
Figure 7.
ICA decomposition of ERP and fMRI joint data for task-irrelevant syllables. Two components that loaded onto the ERP time course in the N1 time-range are shown. The average syllable-related ERP time course is plotted in green and orange. The corresponding estimated jICA-ERP component is plotted in cyan and magenta. The fMRI map is thresholded at z>1.96 for display purposes.
Discussion
In this fMRI-ERP study, working memory load was manipulated in an n-back task while subjects ignored task-irrelevant sounds. Attended and ignored sounds were fixed while memory load varied between blocks. Control blocks consisted of the same task conditions but did not include speech sounds. The main findings complement and extend our recent report of perceptual load effects (Sabri et al., 2013), as well as results in the visual modality as to the dynamic relationship between cognitive control and selective attention: 1. Effects of working memory load on selective attention were observed in the ERPs at 130-210 msec and in auditory cortex localizer-defined ROIs, with higher load resulting in greater irrelevant syllable-related neural response; 2. JICA ERP/fMRI analysis suggests that the ERP component in the N1 time-range is associated primarily with activity in STG and medial prefrontal cortex; 3. The interaction between memory load and presence of irrelevant information revealed stronger activations primarily in frontal and parietal areas due to presence (compared to absence) of irrelevant information in the higher memory load.
The effects of memory load on processing irrelevant speech in auditory cortex were investigated using a localizer scan. The localizer provided a method to create syllable – sensitive ROIs in which contrasts were performed on the irrelevant sounds between task loads. The ROIs were defined in anterior and middle portions of STG on the left. A smaller ROI was identified in the middle STG on the right. High memory load was associated with greater activity for irrelevant syllables in all three ROIs than was low memory load, consistent with greater processing of the syllables and reduced selective attention. These findings are in line with load theory predictions and reports in the visual modality that high memory load promotes processing of distractors such as faces in the fusiform area (de Fockert et al., 2001) and scenes in the parahippocampal place area (Rissman et al., 2009), but inconsistent with those that found no memory load effect (Yi et al., 2004). In Yi's study, a relatively high accuracy on the 2-back memory task may reflect availability of executive function resources to focus on task goals, thereby accounting for the null results. In the current study, accuracy was also relatively high but irrelevant stimulus processing in auditory cortex was greater under high load, suggesting competition between relevant and irrelevant information (Kelley and Lavie, 2011; Lavie and De Fockert, 2005). Recent studies propose that variations in the effects of working memory load on selective attention might depend on additional variables such as individual differences in working memory capacity (Ahmed and de Fockert, 2012a), the level (i.e., local, global) of attended relevant information (Ahmed and de Fockert, 2012b), and the degree of overlap in the cognitive processes necessary for processing of relevant and irrelevant information (Kim et al., 2005).
Differential effects of memory load on selective attention were observed in the N1 ERP response at 130-210 msec. The sources of the N1 were reported previously to include parts of anterior-middle, posterior STG, and medial SFG and IFG depending on sound characteristics and source analyses method (Ahveninen et al., 2011; Jääskeläinen et al., 2004; Ross et al., 2010). Here, ICA of joint ERP and fMRI data for syllable-related responses in NBACK1 and NBACK2 revealed that the N1 is associated primarily with STG and medial prefrontal cortex. Consistent with the fMRI effect, higher working memory load was associated with a larger negative ERP response for task-irrelevant sounds, suggesting again reduced suppression of irrelevant information when cognitive control is already engaged by the ongoing task. These findings are in line with a recent report of working memory effects on attention in a visual dual-task paradigm, starting at 120 msec post stimulus, and with the finding that interference from incongruent flanker stimuli is greater under higher memory demands (Pratt et al., 2011). The temporal pattern of our results is highly similar to that observed with our previous perceptual load manipulation, suggesting that the mental processing required by an ongoing task does not greatly alter the timing of processing irrelevant information at least for the type of sounds used in both studies (Sabri et al., 2013).
Our results are in concordance with predictions of the load model of attention, but also with top-down predictive coding models (Clark, 2013; Feldman and Friston, 2010; Friston and Kiebel, 2009; Garrido et al., 2007; Rao and Ballard, 1999). From a predictive processing perspective, a higher working memory load results in enhanced precision in representing sensory information at lower-levels (i.e., in STG) due to enhanced top-down control (Jiang et al., 2013). As a consequence, deviations from task-relevant sounds produce a larger prediction error observed here as greater activation in auditory cortex in response to task-irrelevant information. Although the load theory of attention similarly predicts a larger response in lower-level sensory regions, the underlying mechanism appears different. It is well established that greater executive control processing is engaged during high memory load tasks (Braver et al., 1997). Load theory accordingly postulates that under high working memory load, executive control mechanisms are not available to maintain current task goals and hence the greater activation observed in sensory cortex to irrelevant stimulation. Whereas load theory predicts a reduced top-down control due to limited cognitive resources, predictive coding assumes enhanced top-down control in order to establish more accurate constraints on expected (relevant) stimuli. In other words, load theory on the one hand assumes a failure of top-down suppression of task irrelevant stimuli at lower sensory levels. Predictive coding, on the other hand, postulates a successful top-down implementation of expected task-relevant stimulus-patterns and an ensuing larger prediction error due to task-irrelevant stimulation. Future investigations will focus on evaluating the role of expectancy in auditory selective attention under load, incorporating functional connectivity analyses (between frontal control and auditory regions) to test the opposing hypotheses regarding top-down control as predicted from the load theory and predictive coding models.
Memory load effects, regardless of presence of irrelevant information, involved both DMN and attention/working memory networks. Modulation of the DMN by memory load has been reported previously, with larger deactivation relative to a resting state as memory load increases (McKiernan et al., 2003). In the current study, higher working memory load (NBACK2) was associated with greater task-induced deactivation predominantly in medial prefrontal cortex, posterior cingulate, and anterior temporal and angular gyri, as well as greater activation in attention prefrontal and superior parietal cortex. As expected irrelevant speech, regardless of memory load, activated regions implicated previously in phonemic processing, including lateral STG and IFG (Liebenthal et al., 2005), overlapping with the extensive activation observed in the high memory load contrast (Syllable_NBACK2 > Control_NBACK2).
The interaction between memory load and irrelevant speech revealed a pattern of stronger activation in STG and various frontal and parietal areas (e.g., SFG, precentral/postcentral gyri, medial SFG, mid-cingulate gyrus, left angular gyrus) under high load in the presence rather than absence of irrelevant information, suggesting involvement of these regions in processing auditory distraction and/or prediction error during tasks that require cognitive control. The medial frontal regions mentioned here have been implicated previously in error detection or conflict monitoring primarily in demanding tasks (Edwards et al., 2012). It is important to note that memory performance was unchanged in the presence of irrelevant speech under low or high memory load. Whether the presence and degree of behavioral interference is a contributing factor remains a question for a future investigation.
Previous studies of working memory modulation of visual processing with both ERPs and fMRI provide evidence that neural suppression of irrelevant information (as opposed to enhancement of relevant information) is the basis for successful memory performance (Rissman et al., 2009; Zanto and Gazzaley, 2009). Strong support for this claim comes from aging research, which suggests that deficits in suppression predict poor working memory ability (Gazzaley et al., 2005). The reduced neural suppression of irrelevant auditory information observed in the current experiment under high working memory demands (associated with poorer performance) is consistent with this literature and is in agreement with the idea of common neural resources for attention and memory (McNab et al., 2008).
Processes of neural enhancement and suppression of auditory information could also explain the effects of perceptual load on selective attention reported previously. Note that perceptual load was not manipulated in the current study. A recent dichotic listening electrocorticogram (ECoG) study demonstrated signal enhancement in auditory cortex under low perceptual load regardless of input relevancy. However, high load (rapid presentation rate) was characterized by enhancement of task-relevant input and sharpening of neural tuning through suppression of non-target sounds (Neelon 2011). Taken together, the neural effects of perceptual and cognitive control demands on auditory selective attention provide strong evidence for the role of ongoing task characteristics in selection.
Highlights.
We examined the neural responses for irrelevant sounds under working memory load.
Higher working memory load was associated with reduced selective attention.
Neural responses for irrelevant sounds were greater in the high memory load.
Load effects were observed at 130-210 ms and in auditory cortex.
Findings are in agreement with the load model of attention predictions.
Acknowledgements
We thank two anonymous reviewers for their valuable comments and suggestions. This work was supported by the National Institute on Deafness and Other Communication Disorders [R03 DC008399]; and the Clinical and Translational Science Award (CTSA) program of the National Center for Research Resources [UL1RR031973].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed L, de Fockert JW. Focusing on attention: the effects of working memory capacity and load on selective attention. PLoS One. 2012a;7:e43101. doi: 10.1371/journal.pone.0043101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed L, de Fockert JW. Working memory load can both improve and impair selective attention: evidence from the Navon paradigm. Attention, perception & psychophysics. 2012b;74:1397–1405. doi: 10.3758/s13414-012-0357-1. [DOI] [PubMed] [Google Scholar]
- Ahveninen J, Hämäläinen M, Jääskeläinen IP, Ahlfors SP, Huang S, Lin F-H, Raij T, Sams M, Vasios CE, Belliveau JW. Attention-driven auditory cortex short-term plasticity helps segregate relevant sounds from noise. Proceedings of the National Academy of Sciences. 2011;108:4182–4187. doi: 10.1073/pnas.1016134108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alain C, Izenberg A. Effects of attentional load on auditory scene analysis. Journal of Cognitive Neuroscience. 2003;15:1063–1073. doi: 10.1162/089892903770007443. [DOI] [PubMed] [Google Scholar]
- Awh E, Vogel EK, Oh SH. Interactions between attention and working memory. Neuroscience. 2006;139:201–208. doi: 10.1016/j.neuroscience.2005.08.023. [DOI] [PubMed] [Google Scholar]
- Bahrami B, Lavie N, Rees G. Attentional Load Modulates Responses of Human Primary Visual Cortex to Invisible Stimuli. Current Biology. 2007;17:509–513. doi: 10.1016/j.cub.2007.01.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AJ, Sejnowski TJ. An information-maximization approach to blind separation and blind deconvolution. Neural Computation. 1995;7:1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bidet-Caulet A.l., Mikyska C, Knight RT. Load effects in auditory selective attention: Evidence for distinct facilitation and inhibition mechanisms. Neuroimage. 2010;50:277–284. doi: 10.1016/j.neuroimage.2009.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage. 1997;5:49–62. doi: 10.1006/nimg.1996.0247. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Adali T, Pearlson GD, Kiehl KA. Neuronal chronometry of target detection: fusion of hemodynamic and event-related potential data. Neuroimage. 2006;30:544–553. doi: 10.1016/j.neuroimage.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Clark A. Whatever next? Predictive brains, situated agents, and the future of cognitive science. Behavioral and Brain Sciences. 2013;36:181–204. doi: 10.1017/S0140525X12000477. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Kincade JM, Shulman GL. Neural systems for visual orienting and their relationships to spatial working memory. Journal of Cognitive Neuroscience. 2002;14:508–523. doi: 10.1162/089892902317362029. [DOI] [PubMed] [Google Scholar]
- Cowan N. Attention and memory: an integrated framework. Oxford University Press; NY, NY: 1998. [Google Scholar]
- Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration of functional MRI. Magnetic Resonance in Medicine. 1999;42:1014–1018. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- de Fockert JW. Beyond perceptual load and dilution: a review of the role of working memory in selective attention. Frontiers in psychology. 2013;4:00287. doi: 10.3389/fpsyg.2013.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Fockert JW, Rees G, Frith CD, Lavie N. The role of working memory in visual selective attention. Science. 2001;291:1803–1806. doi: 10.1126/science.1056496. [DOI] [PubMed] [Google Scholar]
- Edwards BG, Calhoun VD, Kiehl KA. Joint ICA of ERP and fMRI during error-monitoring. Neuroimage. 2012;59:1896–1903. doi: 10.1016/j.neuroimage.2011.08.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman H, Friston K. Attention, uncertainty and free-energy. Frontiers in human neuroscience. 2010;4 doi: 10.3389/fnhum.2010.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K, Kiebel S. Cortical circuits for perceptual inference. Neural Networks. 2009;22:1093–1104. doi: 10.1016/j.neunet.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido MI, Kilner JM, Kiebel SJ, Friston KJ. Evoked brain responses are generated by feedback loops. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20961–20966. doi: 10.1073/pnas.0706274105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Cooney JW, Rissman J, D'Esposito M. Top-down suppression deficit underlies working memory impairment in normal aging. Nature Neuroscience. 2005;8:1298–1300. doi: 10.1038/nn1543. [DOI] [PubMed] [Google Scholar]
- Jääskeläinen IP, Ahveninen J, Bonmassar G, Dale AM, Ilmoniemi RJ, Levänen S, Lin FH, May P, Melcher J, Stufflebeam S, Tiitinen H, Belliveau JW. Human posterior auditory cortex gates novel sounds to consciousness. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6809–6814. doi: 10.1073/pnas.0303760101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Summerfield C, Egner T. Attention sharpens the distinction between expected and unexpected percepts in the visual brain. Journal of Neuroscience. 2013;33:18438–18447. doi: 10.1523/JNEUROSCI.3308-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastner S, Ungerleider LG. Mechanisms of visual attention in the human cortex. Annual Review of Neuroscience. 2000;23:315–341. doi: 10.1146/annurev.neuro.23.1.315. [DOI] [PubMed] [Google Scholar]
- Kelley TA, Lavie N. Working memory load modulates distractor competition in primary visual cortex. Cerebral Cortex. 2011;21:659–665. doi: 10.1093/cercor/bhq139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Kim MS, Chun MM. Concurrent working memory load can reduce distraction. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:16524–16529. doi: 10.1073/pnas.0505454102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavie N. Distracted and confused?: selective attention under load. Trends Cogn Sci. 2005;9:75–82. doi: 10.1016/j.tics.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Lavie N. Attention, Distraction, and Cognitive Control Under Load. Current Directions in Psychological Science. 2010;19:143–148. [Google Scholar]
- Lavie N, De Fockert J. The role of working memory in attentional capture. Psychon Bull Rev. 2005;12:669–674. doi: 10.3758/bf03196756. [DOI] [PubMed] [Google Scholar]
- Lavie N, Hirst A, de Fockert JW, Viding E. Load theory of selective attention and cognitive control. Journal of Experimental Psychology: General. 2004;133:339–354. doi: 10.1037/0096-3445.133.3.339. [DOI] [PubMed] [Google Scholar]
- Liebenthal E, Binder JR, Spitzer SM, Possing ET, Medler DA. Neural substrates of phonemic perception. Cerebral Cortex. 2005;15:1621–1631. doi: 10.1093/cercor/bhi040. [DOI] [PubMed] [Google Scholar]
- Mayer JS, Bittner RA, Nikolifá D, Bledowski C, Goebel R, Linden DEJ. Common neural substrates for visual working memory and attention. Neuroimage. 2007;36:441–453. doi: 10.1016/j.neuroimage.2007.03.007. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. Journal of Cognitive Neuroscience. 2003;15:394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- McNab F, Leroux G.l., Strand F, Thorell L, Bergman S, Klingberg T. Common and unique components of inhibition and working memory: An fMRI, within-subjects investigation. Neuropsychologia. 2008;46:2668–2682. doi: 10.1016/j.neuropsychologia.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Pratt N, Willoughby A, Swick D. Effects of working memory load on visual selective attention: Behavioral and electrophysiological evidence. Frontiers in human neuroscience. 2011;5 doi: 10.3389/fnhum.2011.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RPN, Ballard DH. Predictive coding in the visual cortex: a functional interpretation of some extra-classical receptive-field effects. Nature Neuroscience. 1999;2:79–87. doi: 10.1038/4580. [DOI] [PubMed] [Google Scholar]
- Rees G, Frith CD, Lavie N. Modulating irrelevant motion perception by varying attentional load in an unrelated task. Science. 1997;278:1616–1619. doi: 10.1126/science.278.5343.1616. [DOI] [PubMed] [Google Scholar]
- Rinne T, Koistinen S, Salonen O, Alho K. Task-Dependent Activations of Human Auditory Cortex during Pitch Discrimination and Pitch Memory Tasks. Journal of Neuroscience. 2009;29:13338–13343. doi: 10.1523/JNEUROSCI.3012-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. The effect of non-visual working memory load on top-down modulation of visual processing. Neuropsychologia. 2009;47:1637–1646. doi: 10.1016/j.neuropsychologia.2009.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B, Hillyard SA, Picton TW. Temporal dynamics of selective attention during dichotic listening. Cerebral Cortex. 2010;20:1360–1371. doi: 10.1093/cercor/bhp201. [DOI] [PubMed] [Google Scholar]
- Saad ZS, Glen DR, Chen G, Beauchamp MS, Desai R, Cox RW. A new method for improving functional-to-structural MRI alignment using local Pearson correlation. Neuroimage. 2009;44:839–848. doi: 10.1016/j.neuroimage.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M, Humphries C, Binder JR, Liebenthal E. Neural events leading to and associated with detection of sounds under high processing load. Human Brain Mapping. 2013a;34:587–597. doi: 10.1002/hbm.21457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M, Humphries C, Verber M, Mangalathu J, Desai A, Binder JR, Liebenthal E. Perceptual Demand Modulates Activation of Human Auditory Cortex in Response to Task-irrelevant Sounds. Journal of Cognitive Neuroscience. 2013b;25:1553–1562. doi: 10.1162/jocn_a_00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M, Liebenthal E, Waldron EJ, Medler DA, Binder JR. Attentional modulation in the detection of irrelevant deviance: a simultaneous ERP/fMRI study. Journal of Cognitive Neuroscience. 2006;18:689–700. doi: 10.1162/jocn.2006.18.5.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S, Vuilleumier P, Hutton C, Maravita A, Dolan RJ, Driver J. Attentional load and sensory competition in human vision: modulation of fMRI responses by load at fixation during task-irrelevant stimulation in the peripheral visual field. Cerebral Cortex. 2005;15:770–786. doi: 10.1093/cercor/bhh178. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. Thieme Medical; New York: 1988. [Google Scholar]
- Xu J, Monterosso J, Kober H, Balodis IM, Potenza MN. Perceptual Load-Dependent Neural Correlates of Distractor Interference Inhibition. PLoS One. 2011;6:e14552. doi: 10.1371/journal.pone.0014552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi DJ, Woodman GF, Widders D, Marois R, Chun MM. Neural fate of ignored stimuli: dissociable effects of perceptual and working memory load. Nature Neuroscience. 2004;7:992–996. doi: 10.1038/nn1294. [DOI] [PubMed] [Google Scholar]
- Zanto TP, Gazzaley A. Neural suppression of irrelevant information underlies optimal working memory performance. The Journal of Neuroscience. 2009;29:3059–3066. doi: 10.1523/JNEUROSCI.4621-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanto TP, Rubens MT, Thangavel A, Gazzaley A. Causal role of the prefrontal cortex in top-down modulation of visual processing and working memory. Nature Neuroscience. 2011;14:656–661. doi: 10.1038/nn.2773. [DOI] [PMC free article] [PubMed] [Google Scholar]