Abstract
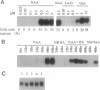
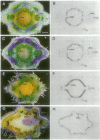
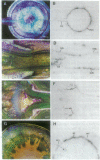
The expression patterns of a cDNA clone, p48h-10, of an auxin-induced gene were examined in isolated mesophyll cells of Zinnia and in the organs of Zinnia plants. In the isolated mesophyll cells, the mRNA accumulates in 48 hr of culture with 1-naphthaleneacetic acid alone. Because the first cell division occurs before 36 hr of culture, the gene probably is not involved in cell division. Benzyladenine does not induce expression of this gene, but the combination of 1-naphthaleneacetic acid and benzyladenine induces the mRNA accumulation about 24 hr earlier than does 1-naphthaleneacetic acid alone. Tissue print hybridization shows that the mRNA is present predominantly in the cambial region in stems, leaves, and roots and in the vascular bundles in flower buds but does not occur in the apical regions of shoot or root. The characteristics of the gene expression, including auxin- and cytokinin-regulated induction and cambial region localization, encourage us to suggest that the gene is involved in the early process of vascular differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainley W. M., McNeil K. J., Hill J. W., Lingle W. L., Simpson R. B., Brenner M. L., Nagao R. T., Key J. L. Regulatable endogenous production of cytokinins up to 'toxic' levels in transgenic plants and plant tissues. Plant Mol Biol. 1993 Apr;22(1):13–23. doi: 10.1007/BF00038992. [DOI] [PubMed] [Google Scholar]
- Bankaitis V. A., Aitken J. R., Cleves A. E., Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990 Oct 11;347(6293):561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Church D. L., Galston A. W. Hormonal induction and antihormonal inhibition of tracheary element differentiation in Zinnia cell cultures. Phytochemistry. 1988;27(8):2435–2439. doi: 10.1016/0031-9422(88)87008-0. [DOI] [PubMed] [Google Scholar]
- Demura T., Fukuda H. Molecular cloning and characterization of cDNAs associated with tracheary element differentiation in cultured Zinnia cells. Plant Physiol. 1993 Nov;103(3):815–821. doi: 10.1104/pp.103.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominov J. A., Stenzler L., Lee S., Schwarz J. J., Leisner S., Howell S. H. Cytokinins and auxins control the expression of a gene in Nicotiana plumbaginifolia cells by feedback regulation. Plant Cell. 1992 Apr;4(4):451–461. doi: 10.1105/tpc.4.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming A. J., Mandel T., Hofmann S., Sterk P., de Vries S. C., Kuhlemeier C. Expression pattern of a tobacco lipid transfer protein gene within the shoot apex. Plant J. 1992 Nov;2(6):855–862. [PubMed] [Google Scholar]
- Fleming A. J., Mandel T., Roth I., Kuhlemeier C. The patterns of gene expression in the tomato shoot apical meristem. Plant Cell. 1993 Mar;5(3):297–309. doi: 10.1105/tpc.5.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fosket D. E. The time course of xylem differentiation and its relation to deoxyribonucleic Acid synthesis in cultured coleus stem segments. Plant Physiol. 1970 Jul;46(1):64–68. doi: 10.1104/pp.46.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H., Komamine A. Direct Evidence for Cytodifferentiation to Tracheary Elements without Intervening Mitosis in a Culture of Single Cells Isolated from the Mesophyll of Zinnia elegans. Plant Physiol. 1980 Jan;65(1):61–64. doi: 10.1104/pp.65.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda H., Komamine A. Establishment of an Experimental System for the Study of Tracheary Element Differentiation from Single Cells Isolated from the Mesophyll of Zinnia elegans. Plant Physiol. 1980 Jan;65(1):57–60. doi: 10.1104/pp.65.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly A. J., Zagotta M. T., White R. A., Chang C., Meeks-Wagner D. R. Identification of genes expressed in the tobacco shoot apex during the floral transition. Plant Cell. 1990 Oct;2(10):963–972. doi: 10.1105/tpc.2.10.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Hagen G., Guilfoyle T. J. Altered morphology in transgenic tobacco plants that overproduce cytokinins in specific tissues and organs. Dev Biol. 1992 Oct;153(2):386–395. doi: 10.1016/0012-1606(92)90123-x. [DOI] [PubMed] [Google Scholar]
- Medford J. I., Elmer J. S., Klee H. J. Molecular cloning and characterization of genes expressed in shoot apical meristems. Plant Cell. 1991 Apr;3(4):359–370. doi: 10.1105/tpc.3.4.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford J. I., Horgan R., El-Sawi Z., Klee H. J. Alterations of Endogenous Cytokinins in Transgenic Plants Using a Chimeric Isopentenyl Transferase Gene. Plant Cell. 1989 Apr;1(4):403–413. doi: 10.1105/tpc.1.4.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina A., Segura A., García-Olmedo F. Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant pathogens. FEBS Lett. 1993 Jan 25;316(2):119–122. doi: 10.1016/0014-5793(93)81198-9. [DOI] [PubMed] [Google Scholar]
- Romano C. P., Hein M. B., Klee H. J. Inactivation of auxin in tobacco transformed with the indoleacetic acid-lysine synthetase gene of Pseudomonas savastanoi. Genes Dev. 1991 Mar;5(3):438–446. doi: 10.1101/gad.5.3.438. [DOI] [PubMed] [Google Scholar]
- Savidge R. A. The role of plant hormones in higher plant cellular differentiation. II. Experiments with the vascular cambium, and sclereid and tracheid differentiation in the pine, Pinus contorta. Histochem J. 1983 May;15(5):447–466. doi: 10.1007/BF01002699. [DOI] [PubMed] [Google Scholar]
- Shininger T. L. Is DNA synthesis required for the induction of differentiation in quiescent root cortical parenchyma? Dev Biol. 1975 Jul;45(1):137–150. doi: 10.1016/0012-1606(75)90247-x. [DOI] [PubMed] [Google Scholar]
- Sossountzov L., Ruiz-Avila L., Vignols F., Jolliot A., Arondel V., Tchang F., Grosbois M., Guerbette F., Miginiac E., Delseny M. Spatial and temporal expression of a maize lipid transfer protein gene. Plant Cell. 1991 Sep;3(9):923–933. doi: 10.1105/tpc.3.9.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk P., Booij H., Schellekens G. A., Van Kammen A., De Vries S. C. Cell-specific expression of the carrot EP2 lipid transfer protein gene. Plant Cell. 1991 Sep;3(9):907–921. doi: 10.1105/tpc.3.9.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoma S., Kaneko Y., Somerville C. A non-specific lipid transfer protein from Arabidopsis is a cell wall protein. Plant J. 1993 Mar;3(3):427–436. doi: 10.1046/j.1365-313x.1993.t01-25-00999.x. [DOI] [PubMed] [Google Scholar]
- Tsuboi S., Osafune T., Tsugeki R., Nishimura M., Yamada M. Nonspecific lipid transfer protein in castor bean cotyledon cells: subcellular localization and a possible role in lipid metabolism. J Biochem. 1992 Apr;111(4):500–508. doi: 10.1093/oxfordjournals.jbchem.a123787. [DOI] [PubMed] [Google Scholar]
- Ye Z. H., Varner J. E. Gene expression patterns associated with in vitro tracheary element formation in isolated single mesophyll cells of Zinnia elegans. Plant Physiol. 1993 Nov;103(3):805–813. doi: 10.1104/pp.103.3.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Z. H., Varner J. E. Tissue-Specific Expression of Cell Wall Proteins in Developing Soybean Tissues. Plant Cell. 1991 Jan;3(1):23–37. doi: 10.1105/tpc.3.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]