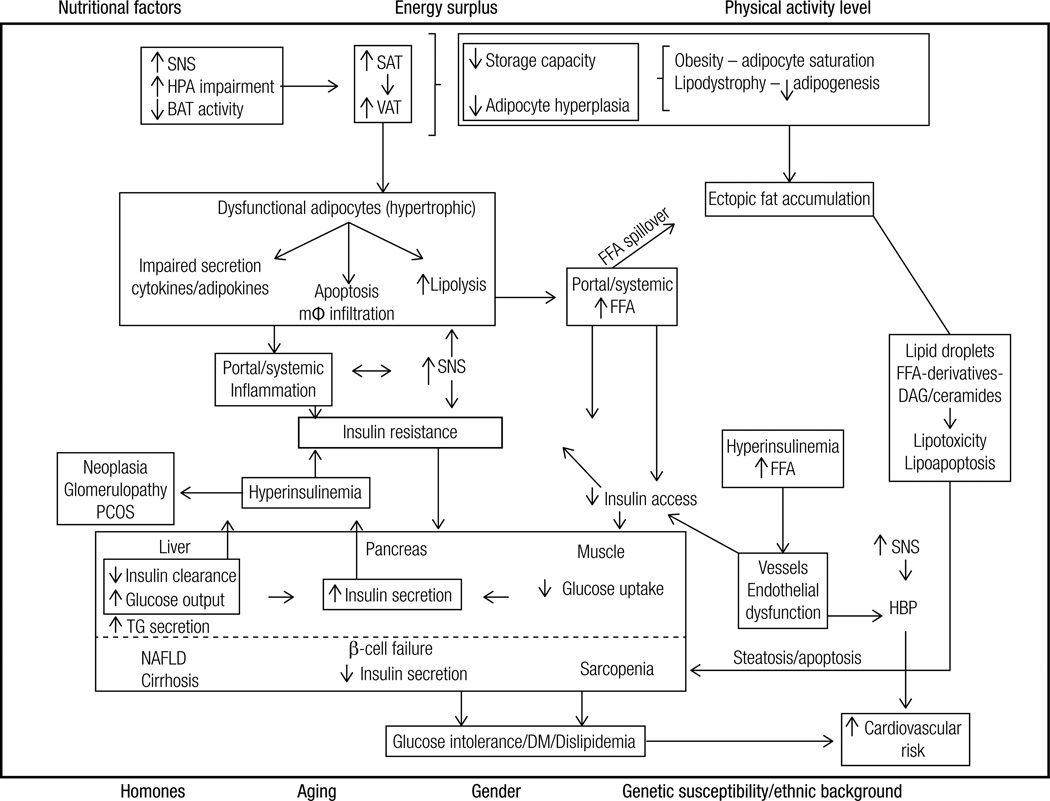
Figure 2. Summary of the pathophysiological mechanisms associated with the development of insulin resistance associated with obesity and comorbidities.
Hormonal status (e.g. menopause), aging, gender, genetic susceptibility and ethnic background interact with lifestyle factors to predispose to the increase of VAT/ectopic fat and the development of IR. Energy surplus secondary to nutritional factors (e.g. high energy food intake) associated with low physical activity levels lead to an increase in SAT/VAT. When the capacity of these tissues to expand becomes saturated (obesity) or limited (lipodystrophy), lipids spill over to non-adipose tissue sites (ectopic fat deposition). Fat growth by hypertrophy generates dysfunctional adipocytes that are more resistant to insulin’s antilipolytic effect and present impaired secretion of cytokines/adipokines (e.g. decreased adiponectin, increased TNFalpha and IL-6). Consequently, FFA and cytokines are released into the circulation. The surplus of FFA to the cells is oxidized, stored (lipids droplets) or metabolized into toxic derivatives (DAG and ceramides). These toxic derivatives lead to insulin resistance, impair cell function (lipotoxicity) or lead to apoptosis (lipoapoptosis). In the pancreas these toxic effects lead to decreased number and impaired capacity of β-cells to secrete insulin, predisposing to the development of type 2 diabetes mellitus; in the liver it leads to non-alcoholic steatohepatitis and subsequently to cirrhosis; in the muscle, to sarcopenia; in the kidney to glomerulopathy etc. Cell dysfunction and death elicit macrophage infiltration, and local and systemic inflammation. In addition, the secretion of inflammatory molecules into the circulation also impairs intracellular insulin signaling. The consequent insulin resistance increases endogenous glucose production by the liver and decreases glucose utilization by peripheral tissues (e.g. muscle). Consequently, glycemia rises and promotes increase in insulin secretion by the pancreas. In addition, hepatic insulin clearance is impaired contributing to hyperinsulinemia, which promotes down regulation of insulin receptor. Among several effects, hyperinsulinemia promotes cell growth (e.g. acanthosis nigricans, neoplasias) and leads to endothelial dysfunction (increased vasoconstriction).
SAT: subcutaneous adipose tissue; VAT: visceral adipose tissue; BAT: brown adipose tissue; FFA: free fatty acids; mφ-macrophage; TG: triglycerides; SNS: sympathetic nervous system; DM: type 2 diabetes mellitus; DAG: diacylglycerol; NAFLD: non-alcoholic fatty liver disease; PCOS: polycystic ovary syndrome; HBP: high blood pressure