Fig 4. IL-15 DCs enhance NK cell cytotoxicity by a cell contact-dependent mechanism involving membrane-bound IL-15.
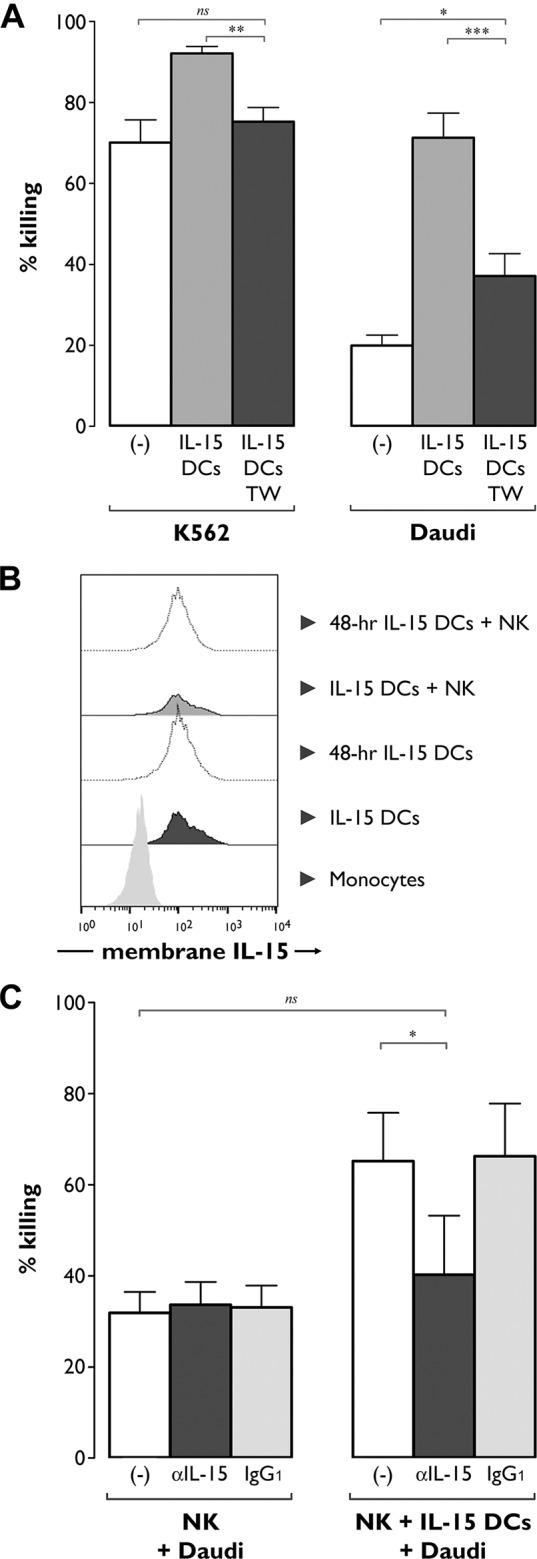
(A) NK cell cytotoxicity was determined after 48-hr co-culture with IL-15 DCs in the absence (grey bars) or presence (TW, dark grey bars) of a Transwell system. NK cells cultured in medium alone for 48 hr ((-), white bars) were used as controls to determine baseline cytotoxicity. K562 and Daudi target cells were added at an E:T ratio of 5:1 and assessed for viability after 4 hr by Annexin-V/PI staining and flow cytometry. Results are expressed as mean (± SEM) percentage killing, which was calculated using the formula specified in “Methods”. Data are from two independent experiments involving 10 different donors (*, P<0.05; **, P<0.01; ***, P<0.001; ns, not significant). (B) Flow cytometric analysis of membrane-bound IL-15 expression on monocytes and mature IL-15 DCs at different time points of culture: at harvest of the DCs (IL-15 DCs), at 48 hr post-harvest (48-hr IL-15 DCs), at the start of IL-15 DC-NK cell co-culture (IL-15 DCs + NK) and after 48 hr of co-culture (48-hr IL-15 DCs + NK). One representative sample out of 3 different donors is shown. (C) NK cells were cultured in the absence (left panel) or presence of autologous IL-15 DCs (right panel) in medium without neutralizing Ab ((-), white bars) or in medium containing either neutralizing anti-IL-15 mAb (αIL15, dark grey bars) or its corresponding isotype control Ab (IgG1, light grey bars). After 16 hr, Daudi tumor cells were added at an E:T ratio of 5:1 for an additional 4 hr after which target cell killing was determined by Annexin-V/PI staining and flow cytometry, as described above. Bars represent mean (± SEM) percentage killing of three individual donors from one experiment (*, P<0.05; ns, not significant).
