Figure 1.
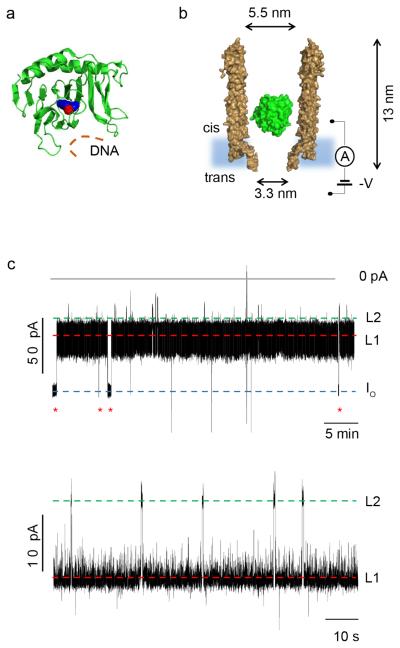
Internalization of AlkB-Fe++ into ClyA-AS. a, Cartoon representation of E. coli AlkB (green) containing a metal ion (Co2+, red sphere) and binding to the cofactor (2-OG, blue spheres). The DNA binding site is depicted by an orange line. PDB_ID 3KHB. b, Representation of a single AlkB-Fe++ enzyme (green) confined in a ClyA-AS nanopore (brown, shown as cross-section) embedded in a planar lipid bilayer (light blue) under a negative applied potential. The dimensions of the pore consider the Van der Waals radii of the atoms. c, Top: Typical current blockades provoked by AlkB-Fe++ molecules (~4 nM, cis) entering a ClyA-AS nanopore at −60 mV. The open pore current (IO) is represented by a blue dashed line, while Level 1 and Level 2 are shown by red and green dashed lines, respectively. The red asterisks represent the restoration of IO upon the exiting of AlkB-Fe++ from the pore. Bottom: Detail of a single AlkB-Fe++ blockade, showing Level 1 (red) and Level 2 (green) current levels. The current traces were collected by applying a Bessel-low pass filter with a 2 kHz cut-off and sampled at 10 kHz. An additional Bessel 8-pole filter with 50 Hz cut-off was digitally applied to the trace shown in c, bottom. All recordings were carried out in 150 mM NaCl, 15 mM Tris HCl pH 8.0, at 28°C, and the AlkB was added to the cis compartment.