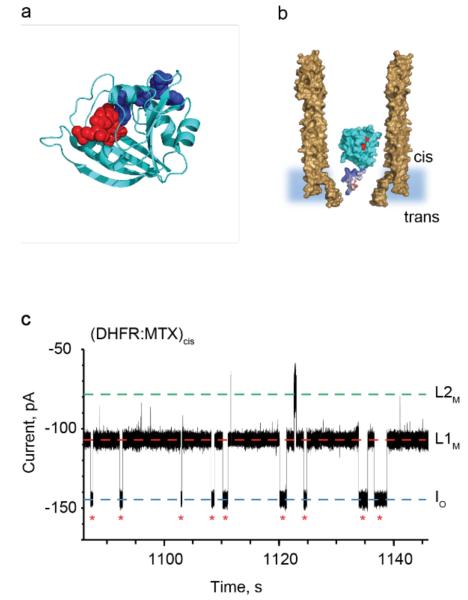
Figure 3.
DHFR as a protein adaptor. a, Cartoon representation of E. coli DHFR (cyan) with bound methotrexate (MTX, red spheres) and NADPH (blue spheres), PDB_ID 1RH3. b, Representation of a single DHFRtag enzyme (cyan) in complex with MTX (red) confined in a ClyA-AS nanopore (brown, shown as cross-section) embedded in a planar lipid bilayer (light blue) under a negative applied potential. The positively charged polypeptide tag added at the C-terminus of DHFR is shown in blue. c, Typical current blockades provoked by the capture of DHFRtag:MTX complexes (20 nM DHFRtag, 400 nM MTX, cis) by the ClyA-AS nanopore at −90 mV. The open pore current (IO) is represented by a blue dashed line, while L1M and L2M are shown by red and green dashed lines, respectively. Red asterisks represent restoration of IO upon the exiting of DHFRtag:MTX from the pore. The current traces were collected in 150 mM NaCl, 15 mM Tris HCl pH 7.5, at 28°C by applying a Bessel-low pass filter with a 2 kHz cut-off and sampled at 10 kHz.