Figure 4.
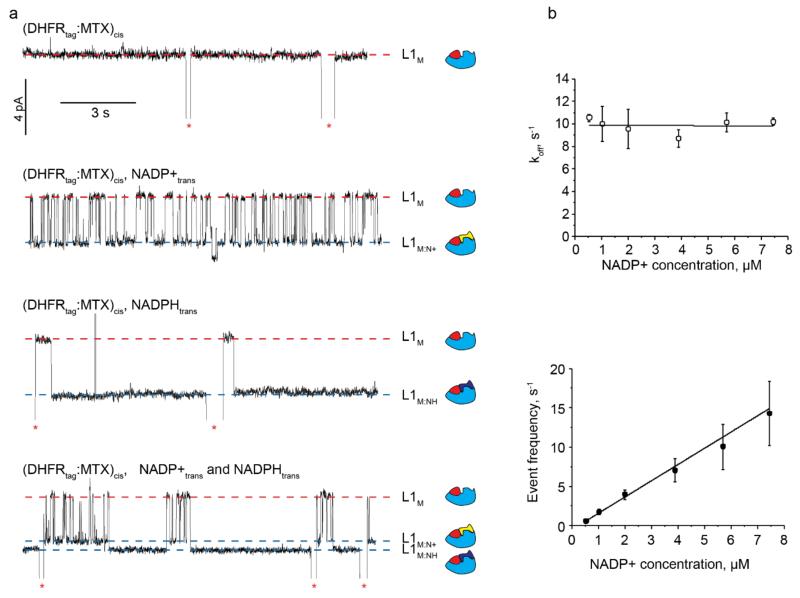
Ligands binding to DHFRtag. a, Ligand-induced current enhancements to individual DHFRtag:MTX blockades at −90 mV. NADP+ and NADPH are added to the trans compartment after addition of 20 nM DHFRtag and 400 nM MTX to the cis compartment. From top to bottom: no ligand; 5.7 μM of NADP+; 0.7 μM of NADPH; 7.4 μM of NADP+ together with 0.7 μM of NADPH. Free and bound Level 1 are shown by red and blue dashed lines, respectively. Red asterisks represent restoration of IO upon the exit of DHFRtag:MTX from the pore. On the right of the current traces is the schematic representation of the interaction of DHFRtag (cyan) with MTX (red), NADP+ (yellow) or NADPH (blue). b, Top: Dissociation rate constants (koff) as a function of the NADP+ concentration added to the trans compartment at −90 mV. Bottom: Event frequency (1/τon) as a function of the NADP+ concentration added to the trans compartment at −90 mV. Errors are shown as standard deviations. All current traces were collected by applying a Bessel-low pass filter with a 2 kHz cut-off and sampled at 10 kHz. An additional Bessel 8-pole filter with 50 Hz cut-off was digitally applied to the traces shown in a. All recordings were carried out in 150 mM NaCl, 15 mM Tris HCl pH 7.5, at 28°C.