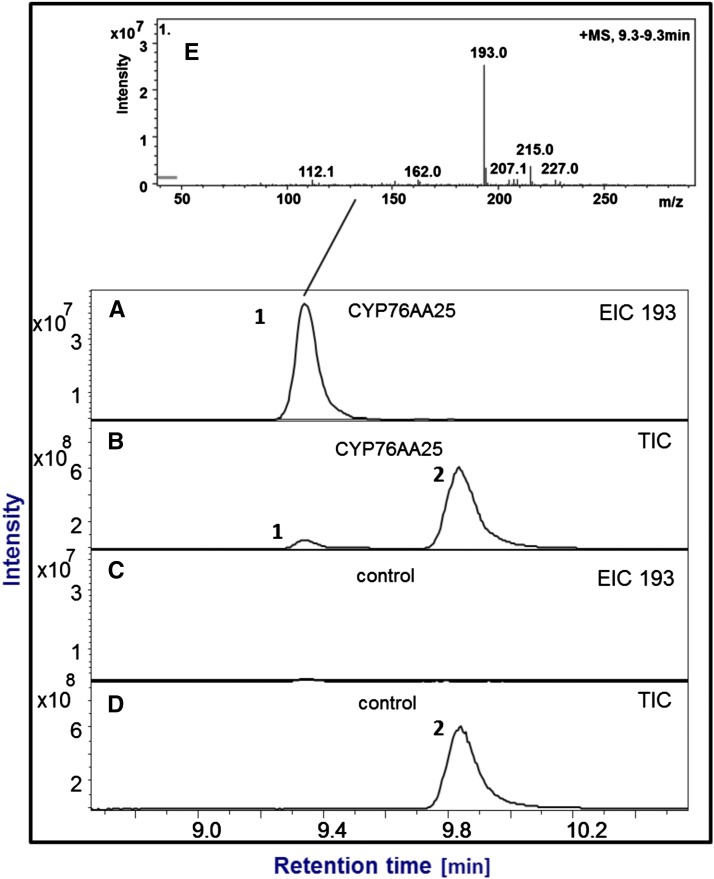
Figure 6.
Isoproturon conversion by CYP76AA25. Shown are total ion chromatograms (TICs) and extracted ion chromatograms (EICs; 193) from liquid chromatography-MS analyses. A and B, Product (1) formed during conversion of isoproturon (2) by CYP76AA25. C and D, Negative control without CYP76AA25. E, Mass spectrum of the product with the major mass ion adducts 193 (M + H) and 215 (M + Na). Assays were performed with 1 h of incubation at 30°C. Reaction product (retention time = 9.32 min) of molecular mass 192 is in agreement with demethylation to N-(4-isopropylphenyl)-N'-methylurea, a known product for CYP450-catalyzed isoproturon conversion (Robineau et al., 1998).