Abstract
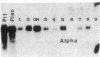
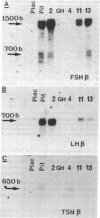
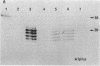
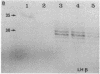
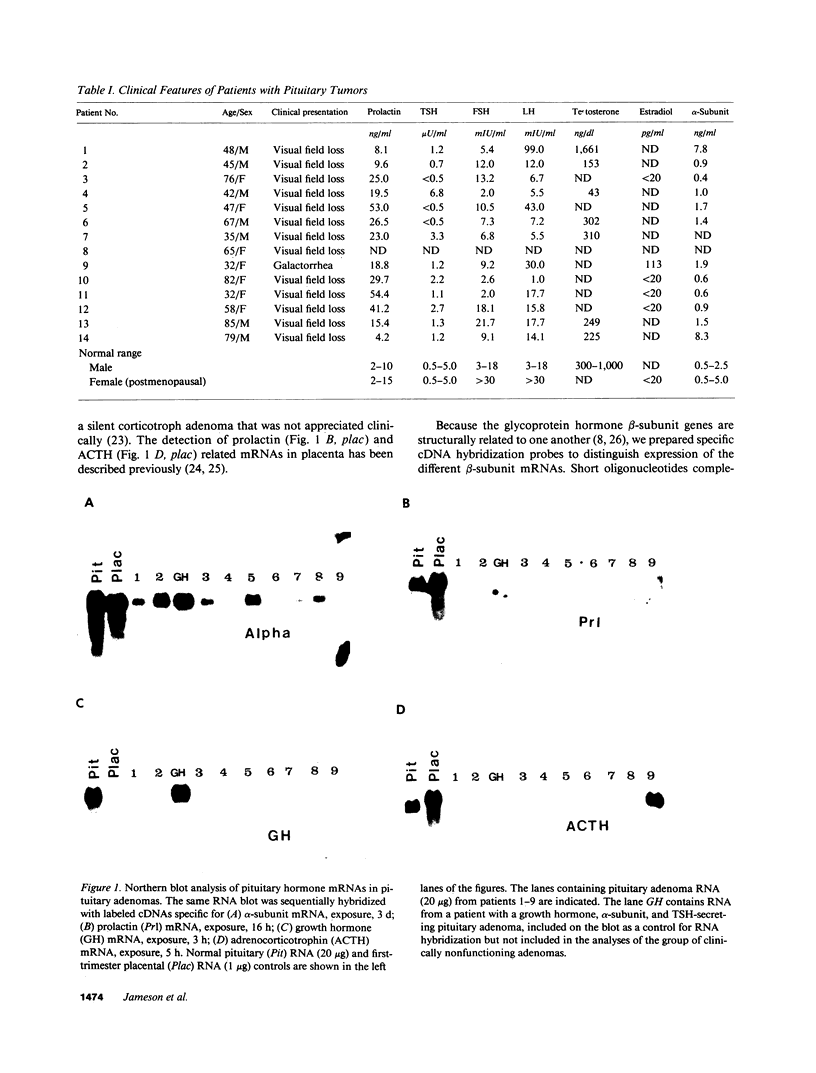
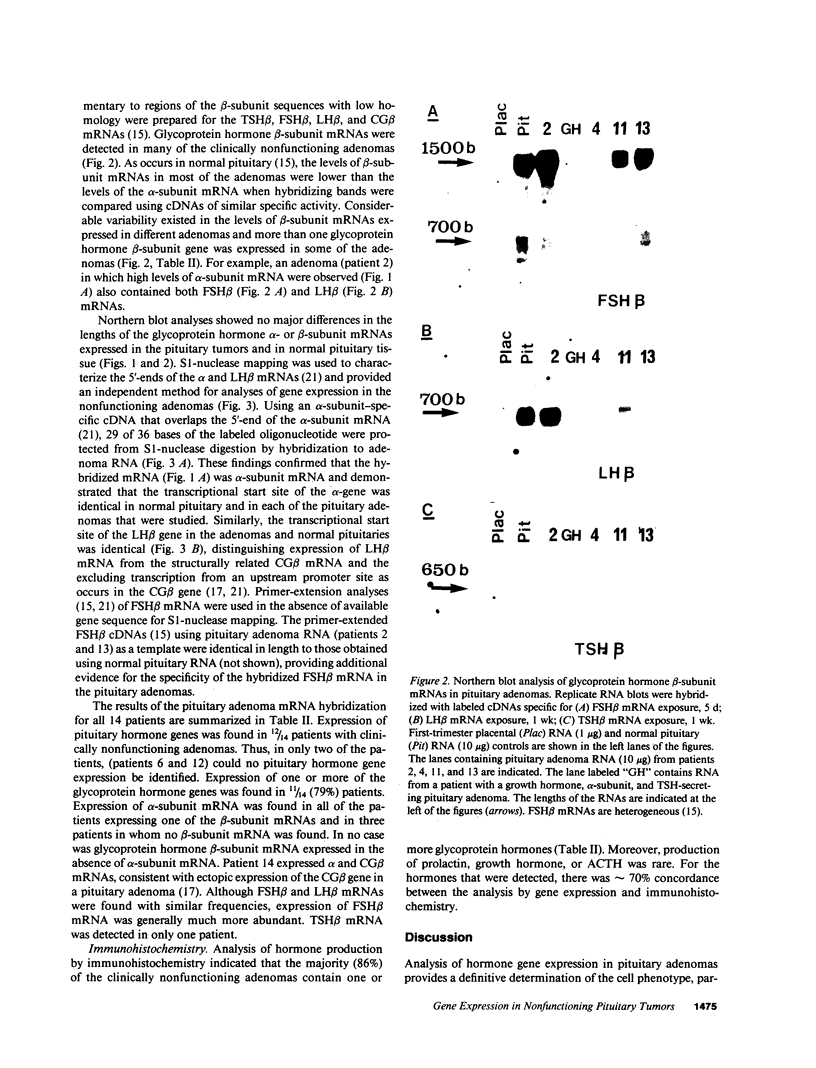
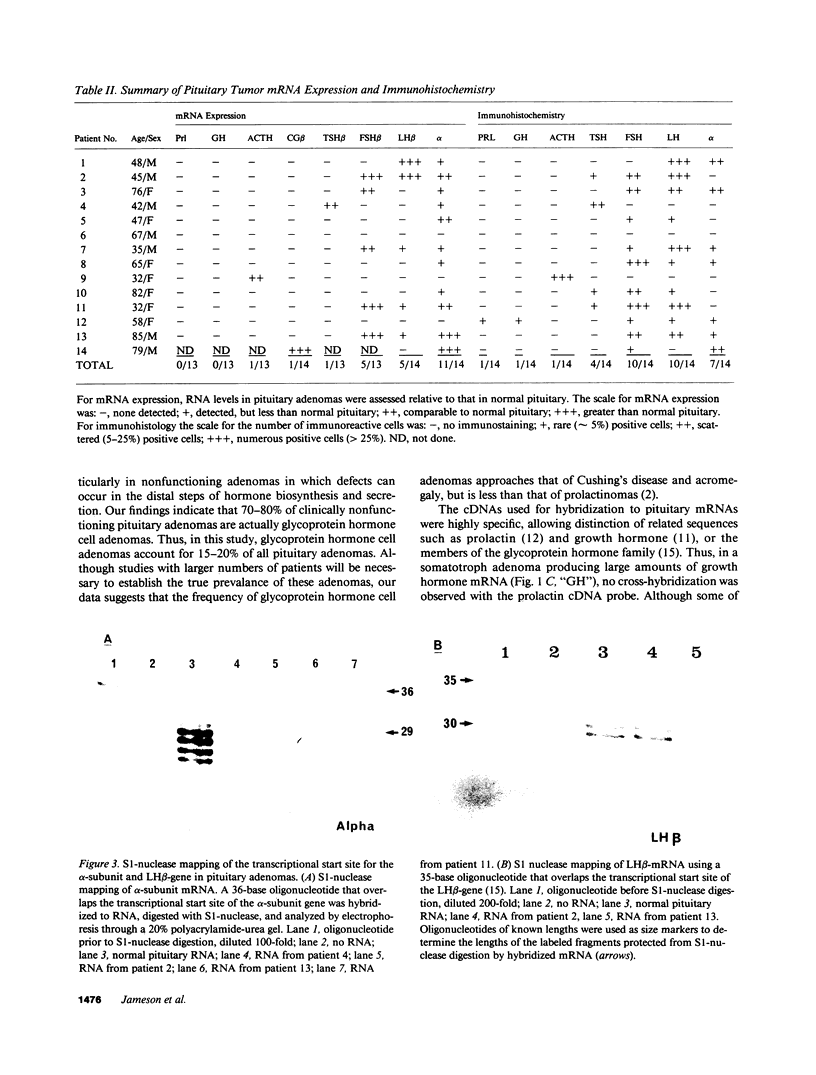
Approximately 25% of patients with pituitary adenomas have no clinical or biochemical evidence for excess hormone secretion and are classified as having null cell or nonfunctioning adenomas. To characterize the cell type of these tumors, we analyzed pituitary hormone gene expression in clinically nonfunctioning pituitary adenomas using specific oligonucleotide probes for the messenger (m)RNAs encoding growth hormone, prolactin, ACTH, and the glycoprotein hormone subunits, alpha, luteinizing hormone (LH)beta, follicle-stimulating hormone (FSH)beta, and thyroid-stimulating hormone (TSH)beta. Expression of one or more of the anterior pituitary hormone genes was found in 12/14 (86%) of the patients with clinically classified nonfunctioning adenomas. Expression of one or more of the glycoprotein hormone genes (alpha, LH beta, FSH beta, TSH beta) was identified most commonly (79%) with expression of multiple beta-subunit genes in many cases. Expression of alpha-subunit mRNA was found in each of the adenomas from patients expressing one of the beta-subunit mRNAs and in three patients with no detectable beta-subunit mRNA. Although FSH beta and LH beta mRNAs were found with similar frequencies in nonfunctioning adenomas, expression of FSH beta mRNA was generally much more abundant. TSH beta mRNA was detected in only one adenoma. The levels of glycoprotein hormone subunit mRNAs were variable in different adenomas, but the lengths of the mRNAs and transcriptional start sites for the alpha- and beta-subunit genes were the same in the pituitary adenomas and in normal pituitary. Growth hormone and prolactin gene expression were not observed in the nonfunctioning adenomas, but ACTH mRNA was found in a single case. Immunohistochemistry of the adenomas confirmed production of one or more pituitary hormones in 13/14 (93%) nonfunctioning tumors, with a distribution of hormone production similar to that of the hormone mRNAs. These data indicate that pituitary adenomas originating from cells producing glycoprotein hormones are common, but are difficult to recognize clinically because of the absence of characteristic endocrine syndromes and defective hormone biosynthesis and secretion.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asa S. L., Gerrie B. M., Singer W., Horvath E., Kovacs K., Smyth H. S. Gonadotropin secretion in vitro by human pituitary null cell adenomas and oncocytomas. J Clin Endocrinol Metab. 1986 May;62(5):1011–1019. doi: 10.1210/jcem-62-5-1011. [DOI] [PubMed] [Google Scholar]
- Berezin M., Olchovsky D., Pines A., Tadmor R., Lunenfeld B. Reduction of follicle-stimulating hormone (FSH) secretion in FSH-producing pituitary adenoma by bromocriptine. J Clin Endocrinol Metab. 1984 Dec;59(6):1220–1223. doi: 10.1210/jcem-59-6-1220. [DOI] [PubMed] [Google Scholar]
- Black P. M., Hsu D. W., Klibanski A., Kliman B., Jameson J. L., Ridgway E. C., Hedley-Whyte E. T., Zervas N. T. Hormone production in clinically nonfunctioning pituitary adenomas. J Neurosurg. 1987 Feb;66(2):244–250. doi: 10.3171/jns.1987.66.2.0244. [DOI] [PubMed] [Google Scholar]
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Normal reference values. N Engl J Med. 1986 Jan 2;314(1):39–49. doi: 10.1056/NEJM198601023140108. [DOI] [PubMed] [Google Scholar]
- Chen C. L., Chang C. C., Krieger D. T., Bardin C. W. Expression and regulation of proopiomelanocortin-like gene in the ovary and placenta: comparison with the testis. Endocrinology. 1986 Jun;118(6):2382–2389. doi: 10.1210/endo-118-6-2382. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooke N. E., Coit D., Shine J., Baxter J. D., Martial J. A. Human prolactin. cDNA structural analysis and evolutionary comparisons. J Biol Chem. 1981 Apr 25;256(8):4007–4016. [PubMed] [Google Scholar]
- DeNoto F. M., Moore D. D., Goodman H. M. Human growth hormone DNA sequence and mRNA structure: possible alternative splicing. Nucleic Acids Res. 1981 Aug 11;9(15):3719–3730. doi: 10.1093/nar/9.15.3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebersold M. J., Quast L. M., Laws E. R., Jr, Scheithauer B., Randall R. V. Long-term results in transsphenoidal removal of nonfunctioning pituitary adenomas. J Neurosurg. 1986 May;64(5):713–719. doi: 10.3171/jns.1986.64.5.0713. [DOI] [PubMed] [Google Scholar]
- Fiddes J. C., Goodman H. M. The gene encoding the common alpha subunit of the four human glycoprotein hormones. J Mol Appl Genet. 1981;1(1):3–18. [PubMed] [Google Scholar]
- Fiddes J. C., Talmadge K. Structure, expression, and evolution of the genes for the human glycoprotein hormones. Recent Prog Horm Res. 1984;40:43–78. doi: 10.1016/b978-0-12-571140-1.50006-2. [DOI] [PubMed] [Google Scholar]
- Hayashizaki Y., Miyai K., Kato K., Matsubara K. Molecular cloning of the human thyrotropin-beta subunit gene. FEBS Lett. 1985 Sep 2;188(2):394–400. doi: 10.1016/0014-5793(85)80409-9. [DOI] [PubMed] [Google Scholar]
- Horvath E., Kovacs K., Killinger D. W., Smyth H. S., Platts M. E., Singer W. Silent corticotropic adenomas of the human pituitary gland: a histologic, immunocytologic, and ultrastructural study. Am J Pathol. 1980 Mar;98(3):617–638. [PMC free article] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Inappropriate secretion of thyroid-stimulating hormone. Ann Intern Med. 1981 Sep;95(3):339–351. doi: 10.7326/0003-4819-95-3-339. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Lindell C. M., Habener J. F. Evolution of different transcriptional start sites in the human luteinizing hormone and chorionic gonadotropin beta-subunit genes. DNA. 1986 Jun;5(3):227–234. doi: 10.1089/dna.1986.5.227. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Lindell C. M., Habener J. F. Gonadotropin and thyrotropin alpha- and beta-subunit gene expression in normal and neoplastic tissues characterized using specific messenger ribonucleic acid hybridization probes. J Clin Endocrinol Metab. 1987 Feb;64(2):319–327. doi: 10.1210/jcem-64-2-319. [DOI] [PubMed] [Google Scholar]
- Jameson J. L., Lindell C. M., Hsu D. W., Habener J. F., Ridgway E. C. Expression of chorionic gonadotropin-beta-like messenger ribonucleic acid in an alpha-subunit-secreting pituitary adenoma. J Clin Endocrinol Metab. 1986 Jun;62(6):1271–1278. doi: 10.1210/jcem-62-6-1271. [DOI] [PubMed] [Google Scholar]
- Kleinberg D. L., Noel G. L., Frantz A. G. Galactorrhea: a study of 235 cases, including 48 with pituitary tumors. N Engl J Med. 1977 Mar 17;296(11):589–600. doi: 10.1056/NEJM197703172961103. [DOI] [PubMed] [Google Scholar]
- Kovacs K., Horvath E., Ryan N., Ezrin C. Null cell adenoma of the human pituitary. Virchows Arch A Pathol Anat Histol. 1980;387(2):165–174. doi: 10.1007/BF00430697. [DOI] [PubMed] [Google Scholar]
- Lee D. W., Markoff E. Synthesis and release of glycosylated prolactin by human decidua in vitro. J Clin Endocrinol Metab. 1986 May;62(5):990–994. doi: 10.1210/jcem-62-5-990. [DOI] [PubMed] [Google Scholar]
- Pierce J. G., Parsons T. F. Glycoprotein hormones: structure and function. Annu Rev Biochem. 1981;50:465–495. doi: 10.1146/annurev.bi.50.070181.002341. [DOI] [PubMed] [Google Scholar]
- Ridgway E. C., Klibanski A., Ladenson P. W., Clemmons D., Beitins I. Z., McArthur J. W., Martorana M. A., Zervas N. T. Pure alpha-secreting pituitary adenomas. N Engl J Med. 1981 May 21;304(21):1254–1259. doi: 10.1056/NEJM198105213042102. [DOI] [PubMed] [Google Scholar]
- Roman S. H., Goldstein M., Kourides I. A., Comite F., Bardin C. W., Krieger D. T. The luteinizing hormone-releasing hormone (LHRH) agonist [D-Trp6-Pro9-NEt]LHRH increased rather than lowered LH and alpha-subunit levels in a patient with an LH-secreting pituitary tumor. J Clin Endocrinol Metab. 1984 Feb;58(2):313–319. doi: 10.1210/jcem-58-2-313. [DOI] [PubMed] [Google Scholar]
- Scheithauer B. W. Surgical pathology of the pituitary: the adenomas. Part II. Pathol Annu. 1984;19(Pt 2):269–329. [PubMed] [Google Scholar]
- Snyder P. J., Bashey H. M., Kim S. U., Chappel S. C. Secretion of uncombined subunits of luteinizing hormone by gonadotroph cell adenomas. J Clin Endocrinol Metab. 1984 Dec;59(6):1169–1175. doi: 10.1210/jcem-59-6-1169. [DOI] [PubMed] [Google Scholar]
- Snyder P. J. Gonadotroph cell adenomas of the pituitary. Endocr Rev. 1985 Fall;6(4):552–563. doi: 10.1210/edrv-6-4-552. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Hakamata Y., Watanabe Y., Kikuno R., Miyata T., Numa S. Complete nucleotide sequence of the human corticotropin-beta-lipotropin precursor gene. Nucleic Acids Res. 1983 Oct 11;11(19):6847–6858. doi: 10.1093/nar/11.19.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge K., Vamvakopoulos N. C., Fiddes J. C. Evolution of the genes for the beta subunits of human chorionic gonadotropin and luteinizing hormone. Nature. 1984 Jan 5;307(5946):37–40. doi: 10.1038/307037a0. [DOI] [PubMed] [Google Scholar]
- Trouillas J., Girod C., Sassolas G., Claustrat B., Lhéritier M., Dubois M. P., Goutelle A. Human pituitary gonadotropic adenoma; histological, immunocytochemical, and ultrastructural and hormonal studies in eight cases. J Pathol. 1981 Dec;135(4):315–336. doi: 10.1002/path.1711350408. [DOI] [PubMed] [Google Scholar]
- Vance M. L., Evans W. S., Thorner M. O. Drugs five years later. Bromocriptine. Ann Intern Med. 1984 Jan;100(1):78–91. doi: 10.7326/0003-4819-100-1-78. [DOI] [PubMed] [Google Scholar]
- Vance M. L., Ridgway E. C., Thorner M. O. Follicle-stimulating hormone- and alpha-subunit-secreting pituitary tumor treated with bromocriptine. J Clin Endocrinol Metab. 1985 Sep;61(3):580–584. doi: 10.1210/jcem-61-3-580. [DOI] [PubMed] [Google Scholar]