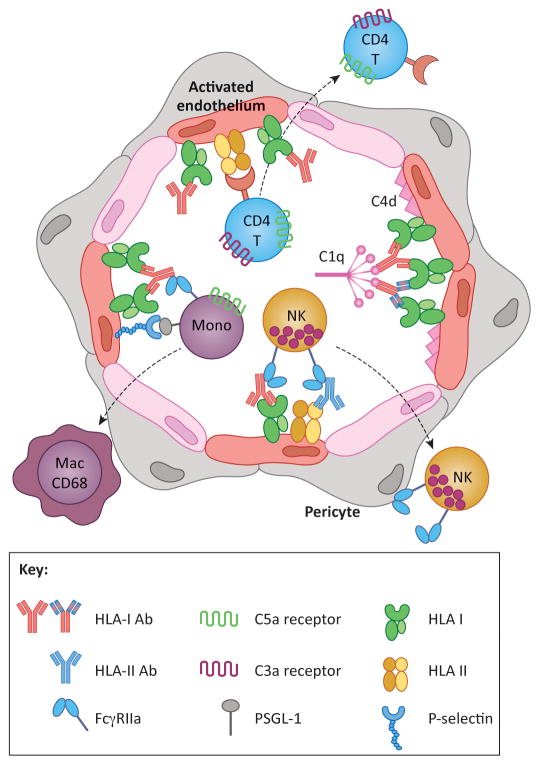
Figure 1. HLA antibodies cause graft injury by inducing phenotypic changes in the donor vasculature.
HLA crosslinking by antibodies of any subclass causes intracellular signaling leading to endothelial cell (EC) activation. Activated ECs express P-selectin, which promotes recruitment of leukocytes via interactions with PSGL-1. Recruited monocytes differentiate into CD68+ macrophages, which can be detected histologically in the capillaries and subendothelial space. Crosslinking of HLA molecules also enhances EC immunogenicity to recipient CD4 T cells, which proliferate and differentiate in response to alloantigen HLA class II. Complement activating antibodies trigger the classical pathway through binding of C1q, resulting in production of anaphylatoxins C3a and C5a, which have the potential to directly augment leukocyte recruitment and T cell alloresponses. Complement activation can be detected by immunohistochemical staining for C4d. Monocytes, neutrophils and NK cells also express FcγRs, which can interact with the heavy chain of HLA antibodies bound to donor ECs. FcγR functions augment leukocyte recruitment, and mediate phagocytosis and antibody-dependent cellular cytotoxicity. Taken together, the pleiotropic functions of HLA antibodies on the allograft ECs cause microvascular inflammation characteristic of antibody-mediated rejection. Antibodies in the figure with the same coloration of the Fc region are of the same subclass, whereas the varied colors within the F(ab′)2 denote unique antigenic specificities.