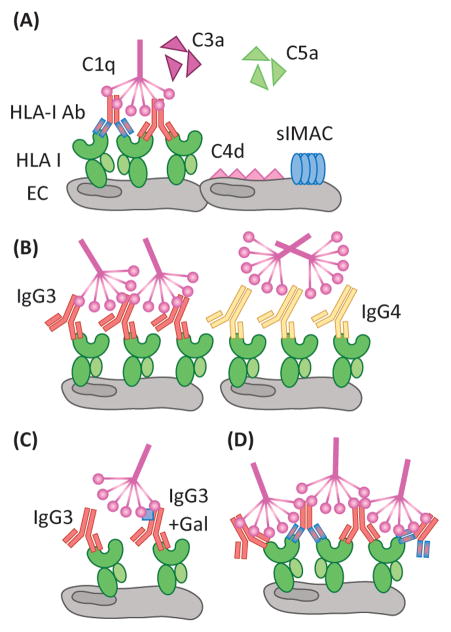
Figure 2. Complement activation by antibody/antigen determinants.
(A) Activation of the classical complement pathway by HLA-Ab is mediated by C1q recognition of the Fc region of IgG. Through a series of subsequent enzymatic cleavages, the complement pathway yields the soluble anaphylatoxins, C3a and C5a, which are potent chemoattractants and stimulators of immune responses. C4d is covalently linked to the cell surface, and is a defining marker of AMR in renal and cardiac transplants. Additionally, sublytic MAC (slMAC), the terminal complex bound to cells but unable to induce lysis, is proving to be an important mediator of endothelial cell (EC) activation. Differences in antibody clonality, as demonstrated by the antibodies of varying specificity (red or blue F(ab′)2 region), allow for increased ratios of IgG:HLA, allowing for more C1q binding. (B) Antibody subclass determines the propensity of C1q binding as IgG3, a prominent complement fixer, is recognized by C1q, whereas the structure of IgG4 makes it a poor C1q binding partner. (C) Differential patterning of the N297 glycan (blue square) of IgG also modulates the level of C1q interaction. Terminal galactose residues confer maximal C1q binding to antibodies. (D) The density of HLA antigen on the surface of the cell, as well as the number of epitopes, heavily dictates the level of complement activation. The proximity of antibody Fc regions is increased when multiple antibodies can bind the same molecule of HLA. Patients with high titer polyclonal DSA may be predisposed to exacerbated complement activation during times of heightened inflammation, such as infection, when HLA expression is increased on the surface of endothelium.