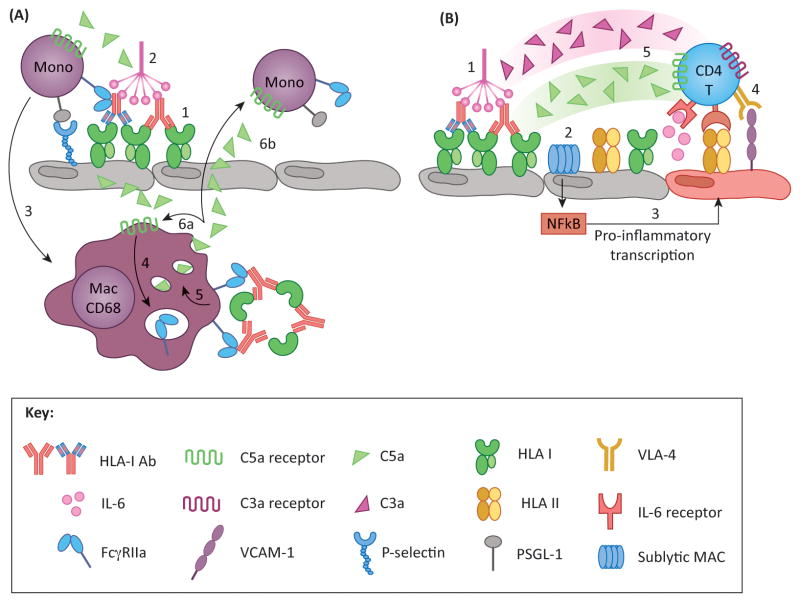
Figure 4. Proposed models of DSA-induced inflammatory loops.
(A) Macrophages perpetuate activation and recruitment via complement and FcγR pathways. DSA crosslinking of HLA on endothelium results in P-selectin mobilization to the cell surface, and provides a binding platform for C1q (1). Classical complement activation produces C5a (2), which has two functions: (i) C5a recruits monocytes to activated EC, which tether to P-selectin via PSGL-1, promoting graft infiltration and differentiation into macrophages (3); and (ii) C5a may act on intragraft CD68+ macrophages and induce FcγR expression (4). These cells can recognize immune complexes (IC) via FcγR, which can upregulate C5a production (5). Newly synthesized C5a may signal in either an autocrine (6a) or paracrine (6b) fashion, mediating further activation of intragraft macrophages and recruitment and activation of monocytes from the periphery, respectively. (B) Recent studies have identified a novel role for endothelial cells and complement in antigen presentation and stimulation of allogeneic T cells. Under inflammatory conditions (such as IFNγ activation) endothelial cells express HLA class II as well as ICAM-1, VCAM-1 and IL-6, molecules that are critical for promoting allo CD4 T cell proliferation (4) and differentiation into Th17 and Treg subsets. Preliminary work has shown that HLA antibodies modulate endothelial alloimmunogenicity through activation of the classical complement pathway (1) resulting in deposition of sublytic MAC (2). MAC triggers non-canonical NFκB signaling leading to inflammatory gene expression (3) and stimulation of allogeneic CD4 T cells (4). T cells also express receptors for complement split products C3a and C5a, which provide costimulatory signals and augment T cell proliferation. Therefore, it is likely that the presence of these anaphylatoxins at the endothelial-T cell interface might enhance T cell alloimmunity (5).