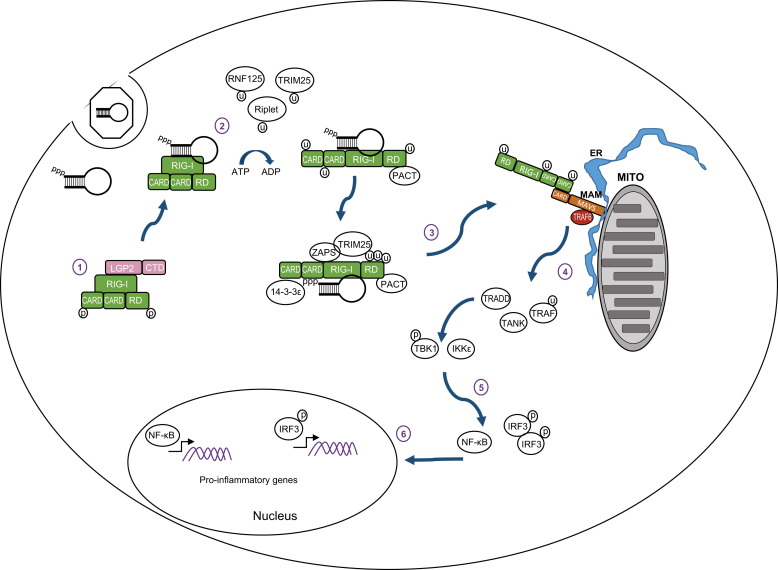
Fig. 1.
RIG-I signaling pathway. (1) In resting cells RIG-I is maintained in a resting state in the cytosol characterized by a ‘closed fist’ conformation with the RD and CARD domains folded over the helicase domain. Interactions with LGP2 may be important for regulating RIG-I. (2) Upon recognition of an RNA ligand containing PAMP motif and exposed 5′ppp RIG-I is ubiquitinated and hydrolyzes ATP to promote a conformational change that holds the ligand PAMP RNA in the RNA binding groove of the RD. (3) Interactions with PACT, ZAPS, TRIM25 and 14-3-3ε lead to formation of a RIG-I translocase that moves from a soluble compartment in the cell to the mitochondrial associated membranes (MAM) where the complex engages MAVS for downstream signaling. The MAM is a specialized extension of outer mitochondria membrane (Mito) and endoplasmic reticulum (ER) that form a synapse to create an innate immune signaling platform. (4) CARD–CARD interactions between RIG-I and MAVS then initiate activation of the MAVS ‘signalosome’ which includes TRAF2, 3, and 6, TRADD, TANK, the IRF3 kinases TBK1 and/or IKKε, and the NF-κB kinase complex. (5) Signaling through these kinases activates IRF3 and NF-κB. (6) Phosphorylated IRF3 and activated NF-κB translocate to the nucleus and initiate transcription of genes encoding products with antiviral, proinflammatory, and immune-regulatory actions.