Abstract
OBJECTIVE
The effects of sex hormones on the immune defenses of the female genital mucosa and its susceptibility to infections are poorly understood. The injectable hormonal contraceptive depot medroxyprogesterone acetate (DMPA) may increase risk of HIV-1 acquisition. We assessed the local concentration in the female genital mucosa of cationic polypeptides with reported antiviral activity in relation to DMPA use.
METHODS
HIV-1-uninfected women were recruited from among couples testing for HIV in Nairobi, Kenya. Cervicovaginal secretion (CVS) samples were collected and the concentrations of HNP1–3, LL-37, lactoferrin, HBD-2 and SLPI were measured by enzyme-linked immunosorbent assays.. Levels of cationic polypeptides in CVS were compared between women who were not using hormonal contraception and those using DMPA, oral, or implantable contraception.
RESULTS
Among 228 women, 165 (72%) reported not using hormonal contraception at enrollment, 41 (18%) used DMPA, 16 (7%) used an oral contraceptive, and 6 (3%) used a contraceptive implant. Compared to non-users of hormonal contraception, DMPA users had significantly higher mean levels of HNP1–3 (2.38 vs. 2.04 log10 ng/ml; p=0.024), LL-37 (0.81 vs. 0.40 log10 ng/ml; p=0.027), and lactoferrin (3.03 vs. 2.60 log10 ng/ml; p=0.002), whereas SLPI and HBD-2 were similar.
CONCLUSIONS
Although all analyzed cationic polypeptides have intrinsic antiviral capacity, their interaction and cumulative effect on female genital mucosa susceptibility to infections in vivo has yet to be unraveled. This study suggests a potential mechanism underlying the effect of DMPA on the innate immune defenses, providing a rationale to investigate its effect on HIV-1 acquisition risk.
Keywords: HIV, cationic, contraceptive, Depo-Provera, progestogen, depot medroxyprogesterone acetate
Introduction
Success of family planning initiatives, particularly in resource limited settings, depends on the wide availability of contraceptive options that are acceptable, highly effective, and safe. These requirements are partially met by long acting injectable hormonal contraceptives, most commonly in the form of depot medroxyprogesterone acetate (DMPA). In sub-Saharan Africa, DMPA accounts for the vast majority of hormonal contraceptive use.1,2 Unfortunately, a growing body of evidence links DMPA use to increased risk of HIV-1 acquisition.3,4 This evidence comes from observational studies, and it remains unknown what proportion of the increased risk, if any, is due to biological effects of DMPA and what proportion is attributable to residual confounding from sexual risk behavior. Identification of biological mechanisms linking DMPA use and risk of HIV infection would increase understanding of whether hormonal contraceptive use is a true biological risk factor for HIV infection.
Innate immune responses in the female genital mucosa are an important component of resistance to infectious agents. Cationic polypeptides are among the principal effector molecules of the innate immune response in the vaginal mucosa.5 In vitro studies show that cationic polypeptides, including the α-defensins human neutrophil peptides 1–3 (HNP1–3), human cathelicidin antimicrobial peptide (hCAP) 18/LL-37, secretory leukocyte protease inhibitor (SLPI), lactoferrin, and human β-defensin (HBD-2), can individually inhibit a number of infectious agents, including HIV-1, through a variety of mechanisms.6–9 These antimicrobial peptides are abundant in cervicovaginal secretions (CVS),10,11 although their interaction in vivo and consequent effect on susceptibility to infections is not fully understood. Despite their intrinsic antiviral activity, higher levels of some cationic polypeptides, particularly HNP1–3 and LL-37, have been associated with increased risk of HIV infection.12 This may be due to the recruitment by cationic polypeptide of CD4+ immune cells, which are preferentially infected by HIV-1.13,14
Changes in antimicrobial peptide levels in the female genital mucosa have been studied mainly in relation to the variation of sex hormones during the menstrual cycle.15 Hormonal contraceptive agents can modulate immune factors as well,16–19 although little work has been done to address their effect locally on the immune defenses of the female genital mucosa.20,21 In this study, we investigated whether DMPA and other forms of hormonal contraception have an effect on the local production of five of the most abundant cationic polypeptides in the female genital mucosa (HNP1–3, LL-37, SLPI, lactoferrin, and HBD-2) by measuring their levels in CVS from HIV-1-uninfected Kenyan women. Studying this relationship may be crucial to understanding how hormonal contraceptives could influence HIV-1 acquisition. Because HIV-1 risk is not uniform across women in the population, we also assessed whether any potential associations between hormonal contraception and these effector molecules differed between women at low-risk of HIV-1 exposure (those in HIV-1 concordant negative couples) and women who are highly exposed to HIV-1 because they are a member of an HIV-1-discordant couple. We chose to include women from HIV-1-discordant couples because they represent a group at particularly high risk of HIV-1 infection who may also be more likely to choose to use hormonal contraception. Their inclusion allowed us to determine if there were any differences between this high risk group and other low risk women in terms of potential associations between hormonal contraceptive use and levels of innate effector molecules.
Methods
Study setting and participants
This study included HIV-1-uninfected women who were recruited from voluntary counseling and testing (VCT) centers in Nairobi, Kenya from 2007 to 2009. These women were members of stable heterosexual couples who attended the VCT center with their male partner. Women included in this study were members of couples that were HIV-1-concordant negative or discordant (male partner infected and female partner uninfected). The women in this study were drawn from a parent cohort described elsewhere.22 Briefly, eligible participants were ≥18 years of age, reported sexual intercourse with their study partner at least three times in the 3 months prior to screening and planned to remain together for the duration of the study (up to 24 months). We excluded couples that were enrolled in another HIV-1 treatment or prevention trial, or planned to be away from Nairobi for 2 consecutive months during follow-up. For discordant couples, we excluded those in which the HIV-1-infected partner was on antiretroviral therapy (ART) or had a history of clinical AIDS (WHO stage IV). Enrolled couples were counseled on condom use, and HIV-1-infected individuals were referred for ART evaluation if their CD4 count met national criteria for treatment initiation. All couples were screened for sexually transmitted infections (STIs) at study entry and treated. For this analysis, women were excluded if they had a hysterectomy, tubal ligation, reported currently having an intrauterine device (IUD), or were missing information on contraceptive use. Written informed consent was obtained from all study participants, and ethical approval was granted by Institutional Review Boards at University of Washington, Karolinska Institutet, and Kenyatta National Hospital.
Assessment of contraceptive use
At enrollment, women completed a sociodemographic questionnaire and provided sexual and reproductive histories. Current contraceptive use was assessed using a standardized questionnaire administered by a study nurse or clinician. Women were asked if they were currently using any form of contraception, and if so, which form(s). Women were categorized as not currently using hormonal contraception, or using DMPA, oral contraception, or implantable hormonal contraception. During the study, implantable contraception available in Kenya was the Jadelle implant, consisting of two levonorgestrel-containing silicone rods inserted under the skin of the upper arm (Bayer Schering Pharma Oy, Berlin-Wedding, Germany).
Collection of cervicovaginal secretion samples
At study enrollment, complete physical and pelvic exams were performed. Samples of CVS were collected by rotating a cotton swab 360° in the cervical os and rotating another swab across the vaginal wall.23 Both swabs were placed in the same tube containing 5 ml sterile phosphate buffered saline and immediately transported to the laboratory on ice. Samples were centrifuged at 800 g for 10 minutes at 4°C and supernatants were separated from the cell pellet and stored at −80°C.
Microbiological testing
HIV-1 rapid testing was conducted in clinic according to Kenyan guidelines using two commercial kits (Determine HIV1/2 Rapid Test, Abbott Laboratories; Bioline Recombigen HIV test, Standard Diagnostics Inc), with confirmation using Vironostika HIV-1 enzyme linked immunosorbant assay (ELISA) (BioMérieux Laboratories). Plasma HIV-1 RNA levels were determined using the Gen-Probe transcription mediated amplification assay, which detects the prevalent HIV-1 subtypes in Kenya, subtypes A, C, and D.24,25 HSV-2 serostatus was determined using the HerpesSelect IgG ELISA kit (Focus Technologies), with an optical density of 1.1–3.4 defined as equivocal and >3.5 defined as positive.26,27 Vaginal gram stains were performed and bacterial vaginosis (BV) was defined as a Nugent score of 7–10.28
Measurement of soluble factors in CVS samples
Concentrations of the cationic polypeptides were quantified by ELISA as previously described.23 Briefly, commercial ELISA kits were used to quantify the immune molecules of interest at the following dilutions: HNP1–3 (1:500), LL-37 (1:10), lactoferrin (1:100–1:200) (Hycult Biotechnology, Uden, The Netherlands), HBD-2 (1:50) (Phoenix Pharmaceutical, Burlingame CA, USA), and SLPI (1:100–1:1000) (R&D Systems, Minneapolis MN, USA). The concentration of the factors in CVS are reported as measured in the original 5 ml dilution. Values that were below the lower limit of detection (LLD) were reported as the midpoint between zero and the LLD. The LLD (pg/ml) was 156.0 for HNP1–3, 100.0 for LL-37, 400 for lactoferrin, 15.6 for HBD-2, and 62.5 for SLPI.
Prostate-specific antigen (PSA) was measured from all CVS samples for this study by an external accredited laboratory (Aleris Medilab, Uppsala, Sweden) using the ARCHITECT Total PSA assay (Abbott Ireland Diagnostics Division, Sligo, Ireland), a chemoluminescent microparticle immunoassay. PSA levels in the CVS are reported as measured in the original 5 ml dilution, and positivity was defined as at least 1 ng/ml, as described previously.29 PSA, a protein secreted during male ejaculation, can be detected in CVS with high sensitivity, and clears from CVS within 48 h with a mean clearance time of approximately 20–27 h, thus presence of PSA indicates recent unprotected sex.29–31
Statistical analysis
Cationic polypeptide concentrations were log10 transformed due to their skewed distribution. Linear regression was used to compare mean log10 polypeptide concentrations between categories of current hormonal contraceptive use (defined as no hormonal contraceptive use, DMPA, oral contraceptives, or implantable hormonal contraception). Other potential correlates, selected a priori, were also assessed. To determine if the relationship between hormonal contraceptive use at polypeptide levels differed between women from HIV-1-discordant and concordant negative couples, we included HIV-1 exposure group as an interaction term in linear regression models. A correlation matrix was calculated to assess the relationships between the cationic polypeptides. Plasma HIV-1 RNA viral levels below the lower limit of detection were set to half the lower limit (75 copies/ml). All analyses were conducted using Stata version 11 (Stata Corp., College Station).
Results
Cohort characteristics by hormonal contraceptive use
We enrolled 246 HIV-1-uninfected women, of whom 233 were eligible for this analysis (women were excluded if they reported a hysterectomy or tubal ligation [n=6], were using an intrauterine device [n=6], or were missing information on contraceptive use [n=1]) and 228 had CVS samples available to determine levels of cationic polypeptides. Of these, 158 were from discordant couples and 70 were from concordant negative couples. At enrollment 165 (72%) reported no current hormonal contraceptive use, 41 (18%) were using DMPA, 16 (7%) were using oral contraception, and 6 (3%) were using an implantable hormonal contraceptive (Table 1). Median age was 28 years [interquartile range (IQR): 24, 33]. Any history of a sexually transmitted infection (STI) was reported by 46 (21%) women, 128 (57%) were HSV-2 seropositive, and 35 (17%) had bacterial vaginosis. Vaginal washing was reported by 61 (26%) women, and 116 (51%) reported using some method to make the vagina drier prior to sex. The CVS from 35 (17%) women had PSA of at least 1 ng/ml, indicating recent unprotected sexual activity. Couples reported a median of 6 sex acts (IQR: 2.5, 9) in the month before enrollment and 92 (40%) reported unprotected sex with their study partner in the last month. Among the women from discordant couples, the median plasma HIV-1 RNA level of their HIV-1 infected partners was 4.8 log10 copies/ml (IQR: 4.1, 5.3).
Table 1.
Demographic and behavioral characteristics of study participants, by hormonal contraceptive use.
| Hormonal Contraception | ||||
|---|---|---|---|---|
| None N=165 |
DMPA N=41 |
Oral N=16 |
Implantable N=6 |
|
| n (%) or median (IQR) | ||||
| Age | 28 (24, 34) | 26 (24, 30) | 27.5 (23, 32.5) | 29 (24, 38) |
| Sex acts in past month | 5 (2.5, 8.5) | 5 (2.5, 8) | 11 (6, 12.25) | 5 (2, 11.5) |
| Any unprotected sex | 64 (38.8) | 16 (39.0) | 12 (75.0) | 0 (0) |
| Vaginal washing | 40 (24.2) | 16 (39.0) | 3 (18.8) | 2 (33.3) |
| Vaginal drying | 77 (46.7) | 25 (61.0) | 11 (68.8) | 3 (50.0) |
| Lifetime STI | 38 (24.1) | 6 (15.0) | 2 (12.5) | 0 (0) |
| HSV-2 seropositive | 94 (57.3) | 21 (52.5) | 8 (53.3) | 5 (83.3) |
| Bacterial vaginosis | 27 (17.1) | 7 (20.0) | 0 (0) | 1 (16.7) |
| Vaginitis | 11 (6.9) | 0 (0) | 0 (0) | 0 (0) |
| Cervicitis | 2 (1.3) | 0 (0) | 0 (0) | 0 (0) |
| Syphilis | 1 (0.6) | 0 (0) | 0 (0) | 0 (0) |
| Trichomoniasis | 5 (3.3) | 2 (5.7) | 0 (0) | 0 (0) |
| Desire additional children | 87 (52.7) | 23 (56.1) | 12 (75.0) | 1 (16.7) |
| Partner viral load (log10 copies/ml) | 4.8 (4.0, 5.4) | 4.6 (4.1, 4.9) | 5.5 (5.2, 5.6) | 5.2 (4.3, 5.3) |
| PSA present | 22 (14.3) | 9 (23.7) | 4 (28.6) | 0 (0) |
| HIV-discordant relationship | 122 (73.9) | 25 (61.1) | 6 (37.5) | 5 (83.3) |
Abbreviations: DMPA, depot medroxyprogesterone acetate; IQR, interquartile range; STI, sexually transmitted infection.
Sociodemographic and behavioral factors were similar between women who were using a form of hormonal contraception (DMPA, oral, or implantable) and those who were not using hormonal contraception; however, women who were not using hormonal contraception had an older median age than DMPA users (28 vs. 26 years, respectively). A larger proportion of women using implants were HSV-2 seropositive (83% in implant users vs. 57% in non-users and 53% among DMPA and oral contraceptive users). Women using oral contraception reported a higher median number of sex acts in the past month compared to other groups (11 vs. 5 sex acts per month, respectively), and women who were using DMPA or oral contraception were more likely to have PSA present in their CVS compared to women who were not using hormonal contraception (24% and 29% vs. 14%, respectively).
Levels of cationic polypeptides
Across all 228 study participants, of the cationic polypeptides measured in CVS samples, lactoferrin (2.72 log10 ng/ml) was found in the highest mean concentration, followed closely by HNP1–3 (2.13 log10 ng/ml). Levels of SLPI (1.92 log10 ng/ml) were lower, and LL-37 (0.51 log10 ng/ml) and HBD-2 (-0.11 log10 ng/ml) were found at the lowest levels. HBD-2 was undetectable in 56 (26%) women, LL-37 was undetectable in 42 (20%) of women, HNP1–3 was undetectable in 9 (4%) women, SLPI was undetectable in 5 (2%) women, and lactoferrin was undetectable in 3 (1%) women. There were significant correlations between HNP1–3, LL-37, lactoferrin, and HBD-2, and a weak correlation between lactoferrin and SLPI (Table 2). The strongest correlations were between lactoferrin and all other peptides. The correlation coefficient between lactoferrin and HNP1–3 was 0.43 (p<0.001), between lactoferrin and LL-37 was 0.39 (p<0.001), between lactoferrin and HBD-2 was 0.37 (p<0.001), and between lactoferrin and SLPI was 0.14 (p=0.040).
Table 2.
The relationship between the levels of cationic polypeptides within individuals is shown in the matrix of Pearson’s correlation coefficients. Values closer to 0 indicate a lack of correlation and values closer to 1 indicate polypeptide levels are more highly correlated within individuals, and a negative coefficient indicates inverse correlation.
| HNP1–3 | LL-37 | SLPI | Lactoferrin | HBD-2 | |
|---|---|---|---|---|---|
| HNP1–3 | 1 | ||||
| LL-37 | 0.50** | 1 | |||
| SLPI | 0.06 | −0.06 | 1 | ||
| Lactoferrin | 0.43** | 0.39** | 0.14* | 1 | |
| HBD-2 | 0.19** | 0.15* | 0.10 | 0.37** | 1 |
Abbreviations: HNP1–3, human neutrophil peptides 1–3; SLPI, secretory leukocyte protease inhibitor; HBD-2, human beta-defensin 2
Log10 transformed
p<0.05
p<0.01
Correlates of cationic polypeptide levels
To identify potential confounding by sociodemographic, behavioral, and biological factors, we investigated correlates of cationic polypeptide concentrations in the study population. Compared to women from discordant couples, those from concordant negative couples had significantly higher mean concentrations of lactoferrin (3.08 vs. 2.57 log10 ng/ml; p<0.001) and HBD-2 (0.28 vs. −0.27 log10 ng/ml; p=0.005) (Table 3). There were no differences between these two groups of women for HNP1–3 (p=0.46), LL-37 (p=0.60), or SLPI (p=0.72). Older age was associated with lower levels of HBD-2 (p=0.004). The levels of HBD-2 were also higher among women who reported a desired for additional children (p=0.014). Unprotected sex in the past month was associated with higher levels of lactoferrin (p<0.001). There were trends indicating that the presence of PSA was associated with higher levels of SLPI (p=0.13) and lactoferrin (p=0.089), and with lower levels of LL-37 (p=0.11). These associations were unaffected by adjustment for contraceptive use (data not shown).
Table 3.
Correlates of cationic peptide concentration in cervicovaginal secretions.
| HNP1–3 Mean (SD) |
Diff. | p | LL-37 Mean (SD) |
Diff. | p | SLPI Mean (SD) |
Diff. | p | Lactoferrin Mean (SD) |
Diff. | P | HBD-2 Mean (SD) |
Diff. | p | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Couple HIV status | |||||||||||||||
| Concordant negative | 2.20 (0.75) | ref | 0.45 (0.93) | ref | 1.95 (1.01) | ref | 3.08 (0.74) | ref | 0.28 (1.20) | ref | |||||
| Discordant | 2.11 (0.86) | −0.09 | 0.46 | 0.53 (1.03) | 0.08 | 0.60 | 1.91 (0.63) | −0.04 | 0.72 | 2.57 (0.51) | −0.50 | <0.001** | −0.27 (1.33) | −0.55 | 0.005** |
| Age | – | −0.003 | 0.70 | – | −0.02 | 0.10 | – | −0.007 | 0.31 | – | −0.008 | 0.22 | – | −0.04 | 0.004** |
| Lifetime STI | |||||||||||||||
| no | 2.16 (0.80) | ref | 0.58 (0.93) | ref | 1.90 (0.79) | ref | 2.74 (0.82) | ref | −0.09 (1.34) | ref | |||||
| yes | 2.05 (0.97) | −0.11 | 0.43 | 0.36 (1.15) | −0.22 | 0.19 | 1.94 (0.68) | 0.05 | 0.71 | 2.69 (0.61) | −0.05 | 0.68 | −0.19 (1.26) | −0.10 | 0.65 |
| HSV-2 | |||||||||||||||
| negative | 2.14 (0.82) | ref | 0.53 (0.97) | ref | 1.87 (0.96) | ref | 2.73 (0.93) | ref | −0.17 (1.37) | ref | |||||
| equivocal | 2.41 (0.33) | 0.27 | 0.26 | 0.95 (0.73) | 0.42 | 0.14 | 2.00 (0.54) | 0.13 | 0.52 | 2.83 (0.62) | 0.10 | 0.62 | 0.37 (1.24) | 0.54 | 0.12 |
| positive | 2.09 (0.88) | −0.05 | 0.67 | 0.43 (1.04) | −0.10 | 0.50 | 1.92 (0.67) | 0.05 | 0.69 | 2.68 (0.69) | −0.04 | 0.72 | −0.13 (1.28) | 0.04 | 0.83 |
| Bacterial vaginosis | |||||||||||||||
| no | 2.15 (0.78) | ref | 0.51 (1.01) | ref | 1.93 (0.74) | ref | 2.66 (0.78) | ref | −0.12 (1.29) | ref | |||||
| yes | 1.91 (1.15) | −0.24 | 0.15 | 0.41 (1.05) | −0.09 | 0.64 | 1.85 (0.74) | −0.07 | 0.59 | 2.78 (0.65) | 0.11 | 0.43 | −0.35 (1.34) | −0.23 | 0.35 |
| Treatable genital infection | |||||||||||||||
| no | 2.11 (0.80) | ref | 0.49 (1.01) | ref | 1.93 (0.76) | ref | 2.63 (0.79) | ref | −0.17 (1.27) | ref | |||||
| yes | 2.06 (1.05) | −0.06 | 0.721 | 0.56 (1.05) | 0.07 | 0.686 | 1.88 (0.70) | −0.05 | 0.687 | 2.87 (0.62) | 0.24 | 0.062 | −0.02 (1.35) | 0.16 | 0.48 |
| Sex acts in past month | – | −0.006 | 0.58 | – | −0.02 | 0.12 | – | 0.02 | 0.016* | – | −0.005 | 0.61 | – | 0.03 | 0.076 |
| Any unprotected sex | |||||||||||||||
| no | 2.10 (0.86) | ref | 0.51 (1.05) | ref | 1.85 (0.71) | ref | 2.53 (0.82) | ref | −0.25 (1.30) | ref | |||||
| yes | 2.20 (0.77) | 0.10 | 0.39 | 0.50 (0.92) | −0.01 | 0.93 | 2.01 (0.83) | 0.16 | 0.12 | 3.00 (0.63) | 0.47 | <0.001** | 0.10 (1.05) | 0.35 | 0.055 |
| Vaginal washing | |||||||||||||||
| no | 2.14 (0.81) | ref | 0.50 (1.01) | ref | 1.89 (0.77) | ref | 2.74 (0.79) | ref | −0.06 (1.31) | ref | |||||
| yes | 2.11 (0.88) | −0.03 | 0.81 | 0.52 (0.98) | 0.01 | 0.94 | 1.98 (0.73) | 0.09 | 0.45 | 2.70 (0.76) | −0.07 | 0.54 | −0.24 (1.34) | −0.18 | 0.38 |
| Vaginal drying | |||||||||||||||
| no | 2.14 (0.79) | ref | 0.61 (0.91) | ref | 1.87 (0.66) | ref | 2.71 (0.91) | ref | −0.11 (1.04) | ref | |||||
| yes | 2.13 (0.87) | −0.01 | 0.90 | 0.40 (1.08) | −0.20 | 0.14 | 1.96 (0.86) | 0.08 | 0.40 | 2.73 (0.62) | 0.02 | 0.88 | −0.10 (0.99) | 0.01 | 0.97 |
| Desire additional children | |||||||||||||||
| no | 2.20 (0.73) | ref | 0.51 (1.02) | ref | 1.91 (0.76) | ref | 2.69 (0.66) | ref | −0.34 (1.32) | ref | |||||
| yes | 2.08 (0.90) | −0.11 | 0.31 | 0.51 (0.98) | 0.002 | 0.99 | 1.92 (0.77) | 0.008 | 0.94 | 2.75 (0.87) | 0.06 | 0.58 | 0.09 (1.29) | 0.43 | 0.015* |
| Partner’s viral load (log10 copies/ml) | – | 0.07 | 0.20 | – | 0.14 | 0.040* | – | −0.005 | 0.90 | – | 0.005 | 0.92 | – | 0.03 | 0.75 |
| PSA present | |||||||||||||||
| no | 2.16 (0.82) | ref | 0.53 (1.02) | ref | 1.89 (0.76) | ref | 2.68 (0.81) | ref | −0.13 (1.31) | ref | |||||
| yes | 1.94 (0.96) | −0.22 | 0.17 | 0.23 (0.99) | −0.302 | 0.11 | 2.10 (0.63) | 0.21 | 0.13 | 2.94 (0.70) | 0.26 | 0.089 | −0.10 (1.41) | 0.03 | 0.92 |
Abbreviations: HIV, human immunodeficiency virus; HSV, herpes simplex virus; PSA, prostate specific antigen; HNP1–3, human neutrophil peptides 1–3; SLPI, secretory leukocyte protease inhibitor; HBD-2, human beta-defensin 2, SD, standard deviation; NA, not applicable
NOTE – Polypeptide concentrations are expressed as log10 ng/ml; Treatable genital infections included bacterial vaginosis, trichomoniasis, syphilis, cervicitis, and vaginitis
p<0.05
p<0.01
Hormonal contraceptive use and cationic peptide levels
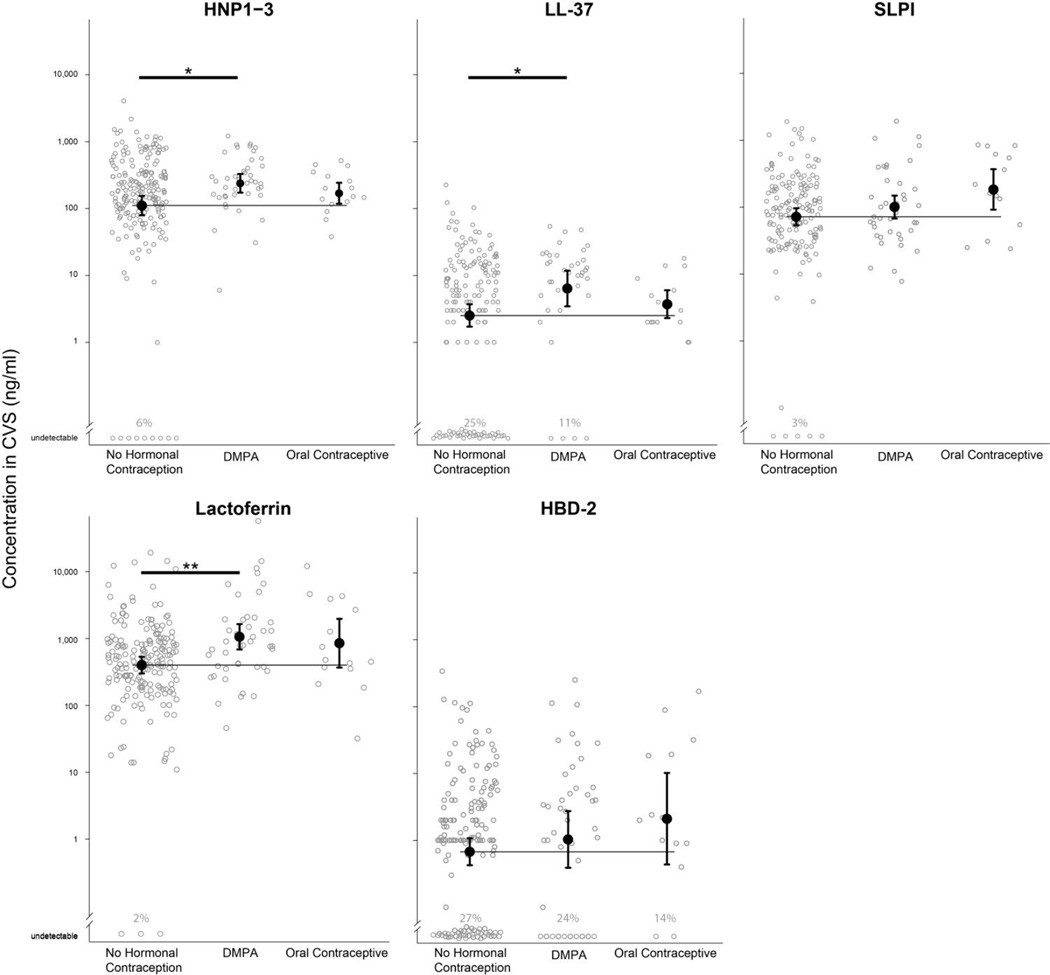
In terms of associations between hormonal contraceptive use with cationic peptide levels, we found no evidence of effect modification by HIV exposure (i.e., HIV-discordant couple versus concordant negative couple), and therefore combined both groups of women in our evaluations of hormonal contraceptive use (electronic supplement 1). Compared to women not using hormonal contraception (n=165), DMPA users (n=41) had significantly higher mean levels of HNP1–3 (2.38 vs. 2.04 log10 ng/ml; p=0.024), LL-37 (0.81 vs. 0.40 log10 ng/ml; p=0.027), and lactoferrin (3.03 vs. 2.60 log10 ng/ml; p=0.002) (Figure 1). These associations were not affected by statistical adjustment for potential confounders (presence of PSA, frequency of sex in the past month, and discordant vs. concordant negative couple) (Table 4). There were no associations between DMPA and the levels of SLPI (p=0.27) or HBD-2 (p=0.43), relative to women not using hormonal contraception.
Figure 1.
Mean concentrations of cationic peptides in cervicovaginal sections (CVS) of HIV-1-uninfected women, stratified by hormonal contraceptive use. Concentrations are expressed as ng/ml. Error bars indicate the 95% confidence interval for the mean estimate. Thin horizontal lines indicate the mean concentration in women using no hormonal contraception. Bold horizontal lines indicate statistical significance at p < 0.05 (*) and p < 0.01 (**). Individuals with peptide levels below the lower limit of detection are indicated at the bottom of each plot. The lower limit of detection for HNP1–3 was 0.156 ng/ml, for LL-37 was 0.100 ng/ml, for SLPI was 0.0625 ng/ml, for lactoferrin was 0.400 ng/ml, and for HBD-2 was 0.0156 ng/ml.
Table 4.
Cationic polypeptide levels in cervicovaginal secretions, by hormonal contraceptive use.
| No Hormonal Mean (SD) |
DMPA Mean (SD) |
Diff. | p | Adj. Diff.a |
Adj. p | Oral Mean (SD) |
Diff. | P | Adj. Diff.a |
Adj. p | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| HNP1–3 | 2.04 (0.92) | 2.38 (0.44) | 0.33 | 0.024* | 0.33 | 0.036* | 2.23 (0.31) | 0.19 | 0.40 | 0.17 | 0.49 |
| LL-37 | 0.40 (1.06) | 0.81 (0.83) | 0.40 | 0.027* | 0.43 | 0.027* | 0.57 (0.41) | 0.17 | 0.53 | 0.28 | 0.35 |
| SLPI | 1.86 (0.82) | 2.00 (0.56) | 0.15 | 0.27 | 0.12 | 0.37 | 2.27 (0.58) | 0.41 | 0.054 | 0.36 | 0.12 |
| Lactoferrin | 2.60 (0.80) | 3.03 (0.62) | 0.42 | 0.002** | 0.36 | 0.010* | 2.93 (0.70) | 0.33 | 0.12 | 0.27 | 0.26 |
| HBD-2 | −0.17 (1.30) | 0.01 (1.38) | 0.18 | 0.43 | 0.27 | 0.26 | 0.33 (1.31) | 0.49 | 0.18 | 0.10 | 0.81 |
Abbreviations: DMPA, depot medroxyprogesterone acetate; HNP1–3, human neutrophil peptides 1–3; SLPI, secretory leukocyte protease inhibitor; HBD-2, human beta-defensin 2; SD, standard deviation; Diff, difference, compared to women using no hormonal contraception; Adj, adjusted value
NOTE – Polypeptide concentrations are expressed as log10 ng/ml
Adjusted for presence of PSA in CVS sample, frequency of sex in the past month, and HIV exposure group (i.e., discordant versus concordant negative couple)
p<0.05
p<0.01
Compared to women not using hormonal contraception, oral contraceptive use (n=16) was not associated with HNP1–3 (p=0.40), LL-37 (p=0.53), or HBD-2 (p=0.18) but there was a trend indicating an association with lactoferrin (p=0.12) (Table 4). Unlike DMPA users, women using oral contraceptives had higher levels of SLPI compared to women not using hormonal contraception, nearly reaching statistical significance (2.27 vs. 1.86 log10 ng/ml; p=0.054).
Women using contraceptive implants (n=6) had significantly higher levels of LL-37 (1.23 vs. 0.40 log10 ng/ml; p=0.040) and lactoferrin (3.34 vs. 2.60 log10 ng/ml; p=0.02), compared to women not using hormonal contraception. There was also a trend indicating implant users had higher levels of HNP1–3 (2.63 vs. 2.04 log10 ng/ml; p=0.087). The point estimates for the difference between implant users and women who were not using hormonal contraception were larger than the corresponding estimates for DMPA users.
Discussion
We found that women who were using DMPA had significantly higher levels of the cationic polypeptides HNP1–3, LL-37, and lactoferrin in their CVS than women not using hormonal contraception. These regulators of the innate immune response in the female genital mucosa may contribute to an increased risk of HIV-1 infection associated with DMPA use. Previous studies have found that DMPA users have an elevated risk of HIV-1 infection;4,32–36 however, not all studies have confirmed this association,3 and those that have may be subject to residual confounding by sexual risk behavior. Therefore, it is important to investigate potential mechanisms to separate the biological and behavioral pathways leading to elevated risk. The use of DMPA involves a large dose of a synthetic progestin administered every three months. While highly effective in preventing pregnancy, the absence of estrogen and persistently high progestin levels induce considerable changes in the genital mucosa. Mechanisms proposed for the increased biological HIV-1 risk associated with DMPA fall into three categories: (1) Changes in vaginal and cervical structure, including cervical ectopy and vaginal thinning, (2) changes in local or systemic immunity, and (3) increased risk of other sexually transmitted infections and detrimental changes in the vaginal flora.21 Any or all of these factors may play a role, but the findings from this study provide increasing evidence that DMPA use has a substantial effect on the innate immune response in the female genital mucosa.
In the context of HIV-1 exposure, HNP1–3, LL-37, and lactoferrin may exert two opposing actions on the innate immune response, the balance of which determines their overall effect on HIV-1 infection risk. While HNP1–3, LL-37, and lactoferrin are all known to have intrinsic antiviral properties (potentially decrease infection risk),8,37–39 HNP1–3 and LL-37 are also potent recruiters of immune cells that are susceptible to HIV-1 infection (potentially increase infection risk).40 Increased LL-37 production has also been shown to enhance HIV-1 transmission by up-regulating HIV co-receptors on Langerhans cells in human skin explants,41 and the active component of DMPA prevents the down-regulation of HIV co-receptors on T cells after activation.19 In agreement with this in vitro evidence, elevated levels of HNP1–3 and LL-37 in CVS have been associated with increased HIV infection risk,12 and DMPA use has been shown to increase numbers of potential HIV target cells and cells expressing activation markers in the human vagina.42 Thus, the observed increase in the production of some cationic polypeptides in the female genital mucosa of DMPA users may provide a mechanistic explanation for an environment that favors mucosal transmission of HIV-1.43
Correlation analysis of the levels of cationic polypeptides in CVS showed that HNP1–3 and LL-37 exhibit the strongest correlation among the five analyzed peptides, consistent with previous findings.44 We also detected a strong correlation between lactoferrin and HNP1–3 as well as between lactoferrin and LL-37. CVS components stem from multiple locations across the female genital tract and are most likely produced by a variety of cell types, upon different stimuli. Nevertheless, the strong correlation observed between HNP1–3, LL-37, and lactoferrin in CVS points towards neutrophils as major producers of these peptides in the female genital mucosa,45 and therefore a potential target of DMPA regulatory effect. In situ analysis of the expression of the three peptides in the mucosa from the upper and lower female genital tract may help to corroborate this hypothesis.
Oral contraceptive use, which involves administration of low doses of estrogen and progesterone analogues, appears to have a qualitatively different relationship with cationic peptide levels compared to DMPA use. Unlike DMPA users, there was little or no evidence that oral contraceptive users differed from non-users of hormonal contraception in their levels of HNP1–3 or LL-37. However, oral contraceptive users had higher levels of SLPI while no such association was found among DMPA users. DMPA and oral contraceptive users were more similar in their elevated levels of lactoferrin compared to non-users of hormonal contraception. In contrast, women using progestogen-based implantable contraception had even higher levels of HNP1–3, LL-37, and lactoferrin compared to DMPA users; however, this was based on a small number of women using implantable contraception (n=6) and it was not possible to adjust for potentially confounding factors. Given the rare but increasing usage of contraceptive implants, more research is needed into their safety in areas of high HIV-1 incidence.
Interestingly, we also found weak evidence that recent unprotected sex, indicated by the presence of PSA in the CVS sample, was associated with lower levels of HNP1–3 and LL-37, and with higher levels of lactoferrin. Significantly higher lactoferrin levels were also found in women who reported any unprotected sex in the past month. The lower concentrations of some of these polypeptides may be due to the immunoregulatory effect of semen in the female genital mucosa.46,47 These observations further emphasize the complex nature of the innate immune response in the genital mucosa, and indicate that the overall effect on HIV-1 risk may be the sum of all factors influencing the local environment at the time of viral exposure.
This observational study adds to existing evidence that DMPA is associated with biological effects that may predispose a woman to HIV-1 infection. However, this cohort experienced a low rate of HIV-1 transmission, with 4 seroconversions (1.9 per 100 woman-years) among women not using hormonal contraception, 2 seroconversions (22.8 per 100 woman-years) among implant users and no seroconversions among DMPA or oral contraceptive users (data not shown). While this study was not designed to evaluate the safety of DMPA, it illustrates that very low rates of HIV-1 transmission can be achieved among high-risk women using DMPA, which we attribute to access to good clinical and counseling resources; this is especially notable since the baseline risk of HIV-1 infection was considerably elevated as all women followed longitudinally had HIV-positive partners. The resources available to women in this study included comprehensive diagnosis and treatment of STIs for both them and their partners, access to unlimited condoms and continued counseling about use of condoms in serodiscordant couples, and access to family planning services. As recent WHO recommendations indicate, the choice of contraceptive methods must be considered against a complex interaction of factors including fertility desire, relationship dynamics, reproductive health, and HIV-1 risk.48 There is a considerable need to improve the available options for contraception, particularly in sub-Saharan Africa, but in the near future DMPA will continue to be an important component of the method mix. Appropriate resources are needed to meet women’s contraceptive needs while minimizing their risk of both HIV-1 infection and unintended pregnancies. Expansion of antiretroviral therapy, including ‘treatment as prevention,’ could make DMPA safer among women at increased risk of HIV acquisition.
This was a relatively large study of HIV-1-uninfected women, including those who were and were not exposed to HIV-1 by an infected male partner. Samples of CVS were collected by swab under consistent conditions in the context of a larger cohort study. Despite these strengths, some limitations exist that affect our interpretation of these data. Hormonal contraceptive use was self-reported. Any resulting misclassification is unlikely to be related to peptide levels, or correlates of peptide levels, and therefore the effect is likely an underestimate of the true associations. The process of collecting cervicovaginal swabs is subject to some variability that decreases the precision of the peptide measurements. Furthermore, we were unable to account for variations in peptide levels throughout the menstrual cycle. Again, the likely effect would be an underestimate of associations. We found some evidence that not all forms of hormonal contraception (e.g., DMPA, oral contraception, and implantable contraception) have the same associations with innate polypeptide peptide levels, but the relatively small number of women who were using oral and implantable contraception limited our ability to explore these differences. We could not distinguish endogenous peptides from peptides originating from semen; however, recent unprotected sex, as measured by the presence of PSA, was associated with lower levels of HNP1–3 and LL-37, indicating these peptides were unlikely to originate from semen. Furthermore, statistical adjustment for the presence of PSA did not affect the observed associations. Finally, we were unable to directly assess the effect of innate peptide levels on HIV-1 risk due to the very low rate of HIV-1 transmission in this cohort.22
Conclusions
We found that DMPA use was associated with higher concentrations of cationic polypeptides in the female genital mucosa. Some of these peptides have been found previously to recruit immune cells and are associated with increased risk of HIV-1 infection. Progestin-based contraception may upregulate these polypeptides in the female genital mucosa, which could result in local recruitment of cells that are susceptible to HIV infection. This may provide independent evidence supporting a biological mechanism for increased HIV-1 acquisition seen with DMPA.
Supplementary Material
Acknowledgments
Funding: Research support included National Institutes of Health (NIH) R01 AI0684316 and R24 TW007988 from Fogarty International Center (FIC) through Vanderbilt University. In addition, CF received support from NIH K24 AI087399, RB was a scholar in the University of Washington International AIDS Research and Training Program (IARTP) funded by FIC D43 TW000007, and BLG was supported by an NIH/FIC postdoctoral fellowship 3D43TW000007-22S1 and by a NIH/NIAID Career Development Award 1K01AI098527. AR was supported by a NIH/NICHD Career Development Award 1K23HD071788. RYC received support from the International Research Scientist Development Award K01 TW008406. KB received support from SIDA/SAREC, and the Swedish Research Council. This publication was made possible with help from the University of Washington Center for AIDS Research (CFAR), an NIH funded program (P30 AI027757), which is supported by the following NIH Institutes and Centers (NIAID, NCI, NIMH, NIDA, NICHD, NHLBI, NIA). The contents of this manuscript are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health, Fogarty International Center or Vanderbilt University.
Footnotes
Meetings at which parts of the data have been presented: Conference on Retroviruses and Opportunistic Infections, March 3 to March 6, 2014. Boston, Massachusetts.
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Hubacher D, Olawo A, Manduku C, Kiarie J, Chen PL. Preventing unintended pregnancy among young women in Kenya: prospective cohort study to offer contraceptive implants. Contraception. 2012 Nov;86(5):511–517. doi: 10.1016/j.contraception.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Sibeko S, Baxter C, Yende N, Karim QA, Karim SS. Contraceptive choices, pregnancy rates, and outcomes in a microbicide trial. Obstetrics and gynecology. 2011 Oct;118(4):895–904. doi: 10.1097/AOG.0b013e31822be512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013 Sep;13(9):797–808. doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 4.Heffron R, Donnell D, Rees H, et al. Use of hormonal contraceptives and risk of HIV-1 transmission: a prospective cohort study. Lancet Infect Dis. 2012 Jan;12(1):19–26. doi: 10.1016/S1473-3099(11)70247-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hancock RE. Cationic peptides: effectors in innate immunity and novel antimicrobials. Lancet Infect Dis. 2001 Oct;1(3):156–164. doi: 10.1016/S1473-3099(01)00092-5. [DOI] [PubMed] [Google Scholar]
- 6.Cole AM, Cole AL. Antimicrobial polypeptides are key anti-HIV-1 effector molecules of cervicovaginal host defense. American journal of reproductive immunology. 2008 Jan;59(1):27–34. doi: 10.1111/j.1600-0897.2007.00561.x. [DOI] [PubMed] [Google Scholar]
- 7.Hazrati E, Galen B, Lu W, et al. Human alpha- and beta-defensins block multiple steps in herpes simplex virus infection. Journal of Immunology. 2006 Dec 15;177(12):8658–8666. doi: 10.4049/jimmunol.177.12.8658. [DOI] [PubMed] [Google Scholar]
- 8.Bergman P, Walter-Jallow L, Broliden K, Agerberth B, Soderlund J. The antimicrobial peptide LL-37 inhibits HIV-1 replication. Current HIV Research. 2007 Jul;5(4):410–415. doi: 10.2174/157016207781023947. [DOI] [PubMed] [Google Scholar]
- 9.Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nature Reviews Immunology. 2006 Jun;6(6):447–456. doi: 10.1038/nri1860. [DOI] [PubMed] [Google Scholar]
- 10.Valore EV, Park CH, Igreti SL, Ganz T. Antimicrobial components of vaginal fluid. American journal of obstetrics and gynecology. 2002 Sep;187(3):561–568. doi: 10.1067/mob.2002.125280. [DOI] [PubMed] [Google Scholar]
- 11.Cole AM. Innate host defense of human vaginal and cervical mucosae. Current topics in microbiology and immunology. 2006;306:199–230. [PubMed] [Google Scholar]
- 12.Levinson P, Kaul R, Kimani J, et al. Levels of innate immune factors in genital fluids: association of alpha defensins and LL-37 with genital infections and increased HIV acquisition. AIDS. 2009 Jan 28;23(3):309–317. doi: 10.1097/QAD.0b013e328321809c. [DOI] [PubMed] [Google Scholar]
- 13.Wang Y, Abel K, Lantz K, Krieg AM, McChesney MB, Miller CJ. The Toll-like receptor 7 (TLR7) agonist, imiquimod, and the TLR9 agonist, CpG ODN, induce antiviral cytokines and chemokines but do not prevent vaginal transmission of simian immunodeficiency virus when applied intravaginally to rhesus macaques. Journal of Virology. 2005 Nov;79(22):14355–14370. doi: 10.1128/JVI.79.22.14355-14370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang ZQ, Wietgrefe SW, Li Q, et al. Roles of substrate availability and infection of resting and activated CD4+ T cells in transmission and acute simian immunodeficiency virus infection. Proceedings of the National Acadamy of Sciences U S A. 2004 Apr 13;101(15):5640–5645. doi: 10.1073/pnas.0308425101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. American journal of reproductive immunology. 2011 Mar;65(3):196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes GC, Clark EA. Regulation of dendritic cells by female sex steroids: relevance to immunity and autoimmunity. Autoimmunity. 2007 Sep;40(6):470–481. doi: 10.1080/08916930701464764. [DOI] [PubMed] [Google Scholar]
- 17.Hughes GC, Thomas S, Li C, Kaja MK, Clark EA. Cutting edge: progesterone regulates IFN-alpha production by plasmacytoid dendritic cells. Journal of Immunology. 2008 Feb 15;180(4):2029–2033. doi: 10.4049/jimmunol.180.4.2029. [DOI] [PubMed] [Google Scholar]
- 18.Hughes GC, Clark EA, Wong AH. The intracellular progesterone receptor regulates CD4+ T cells and T cell-dependent antibody responses. Journal of leukocyte biology. 2013 Mar;93(3):369–375. doi: 10.1189/jlb.1012491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huijbregts RP, Helton ES, Michel KG, et al. Hormonal contraception and HIV-1 infection: medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology. 2013 Mar;154(3):1282–1295. doi: 10.1210/en.2012-1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hel Z, Stringer E, Mestecky J. Sex steroid hormones, hormonal contraception, and the immunobiology of human immunodeficiency virus-1 infection. Endocrine reviews. 2010 Feb;31(1):79–97. doi: 10.1210/er.2009-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blish CA, Baeten JM. Hormonal contraception and HIV-1 transmission. American journal of reproductive immunology. 2011 Mar;65(3):302–307. doi: 10.1111/j.1600-0897.2010.00930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guthrie BL, Lohman-Payne B, Liu AY, et al. HIV-1-specific enzyme-linked immunosorbent spot assay responses in HIV-1-exposed uninfected partners in discordant relationships compared to those in low-risk controls. Clinical and Vaccine Immunology. 2012 Nov;19(11):1798–1805. doi: 10.1128/CVI.00179-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levinson P, Choi RY, Cole AL, et al. HIV-neutralizing activity of cationic polypeptides in cervicovaginal secretions of women in HIV-serodiscordant relationships. PLoS ONE. 2012;7(2):e31996. doi: 10.1371/journal.pone.0031996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeVange Panteleeff D, Emery S, Richardson BA, et al. Validation of performance of the gen-probe human immunodeficiency virus type 1 viral load assay with genital swabs and breast milk samples. Journal of Clinical Microbiology. 2002 Nov;40(11):3929–3937. doi: 10.1128/JCM.40.11.3929-3937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emery S, Bodrug S, Richardson BA, et al. Evaluation of performance of the Gen-Probe human immunodeficiency virus type 1 viral load assay using primary subtype A, C, and D isolates from Kenya. Journal of Clinical Microbiology. 2000 Jul;38(7):2688–2695. doi: 10.1128/jcm.38.7.2688-2695.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Golden MR, Ashley-Morrow R, Swenson P, Hogrefe WR, Handsfield HH, Wald A. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sexually Transmitted Diseases. 2005 Dec;32(12):771–777. doi: 10.1097/01.olq.0000175377.88358.f3. [DOI] [PubMed] [Google Scholar]
- 27.Muiru AN, Guthrie BL, Bosire R, et al. Incident HSV-2 Infections Are Common Among HIV-1-discordant Couples. Journal of Infectious Diseases. 2013 Jul 21; doi: 10.1093/infdis/jit303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nugent RP, Krohn MA, Hillier SL. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. Journal of Clinical Microbiology. 1991 Feb;29(2):297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macaluso M, Lawson L, Akers R, et al. Prostate-specific antigen in vaginal fluid as a biologic marker of condom failure. Contraception. 1999 Mar;59(3):195–201. doi: 10.1016/s0010-7824(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 30.Gallo MF, Behets FM, Steiner MJ, et al. Prostate-specific antigen to ascertain reliability of self-reported coital exposure to semen. Sexually Transmitted Diseases. 2006 Aug;33(8):476–479. doi: 10.1097/01.olq.0000231960.92850.75. [DOI] [PubMed] [Google Scholar]
- 31.Graves HC, Sensabaugh GF, Blake ET. Postcoital detection of a male-specific semen protein. Application to the investigation of rape. New England Journal of Medicine. 1985 Feb 7;312(6):338–343. doi: 10.1056/NEJM198502073120603. [DOI] [PubMed] [Google Scholar]
- 32.Wand H, Ramjee G. The effects of injectable hormonal contraceptives on HIV seroconversion and on sexually transmitted infections. AIDS. 2012 Jan 28;26(3):375–380. doi: 10.1097/QAD.0b013e32834f990f. [DOI] [PubMed] [Google Scholar]
- 33.Baeten JM, Benki S, Chohan V, et al. Hormonal contraceptive use herpes simplex virus infection, and risk of HIV-1 acquisition among Kenyan women. AIDS. 2007 Aug 20;21(13):1771–1777. doi: 10.1097/QAD.0b013e328270388a. [DOI] [PubMed] [Google Scholar]
- 34.Feldblum PJ, Lie CC, Weaver MA, et al. Baseline factors associated with incident HIV and STI in four microbicide trials. Sexually Transmitted Diseases. 2010 Oct;37(10):594–601. [PubMed] [Google Scholar]
- 35.Ungchusak K, Rehle T, Thammapornpilap P, Spiegelman D, Brinkmann U, Siraprapasiri T. Determinants of HIV infection among female commercial sex workers in northeastern Thailand: results from a longitudinal study. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology. 1996 Aug 15;12(5):500–507. doi: 10.1097/00042560-199608150-00010. [DOI] [PubMed] [Google Scholar]
- 36.Kumwenda NI, Kumwenda J, Kafulafula G, et al. HIV-1 incidence among women of reproductive age in Malawi. International Journal of STD and AIDS. 2008 May;19(5):339–341. doi: 10.1258/ijsa.2007.007165. [DOI] [PubMed] [Google Scholar]
- 37.Harmsen MC, Swart PJ, de Bethune MP, et al. Antiviral effects of plasma and milk proteins: lactoferrin shows potent activity against both human immunodeficiency virus and human cytomegalovirus replication in vitro. Journal of Infectious Diseases. 1995 Aug;172(2):380–388. doi: 10.1093/infdis/172.2.380. [DOI] [PubMed] [Google Scholar]
- 38.McNeely TB, Dealy M, Dripps DJ, Orenstein JM, Eisenberg SP, Wahl SM. Secretory leukocyte protease inhibitor: a human saliva protein exhibiting anti-human immunodeficiency virus 1 activity in vitro. The Journal of Clinical Investigation. 1995 Jul;96(1):456–464. doi: 10.1172/JCI118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang TL, Vargas J, Jr, DelPortillo A, Klotman ME. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. The Journal of Clinical Investigation. 2005 Mar;115(3):765–773. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chertov O, Michiel DF, Xu L, et al. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. The Journal of biological chemistry. 1996 Feb 9;271(6):2935–2940. doi: 10.1074/jbc.271.6.2935. [DOI] [PubMed] [Google Scholar]
- 41.Ogawa Y, Kawamura T, Matsuzawa T, et al. Antimicrobial peptide LL-37 produced by HSV-2-infected keratinocytes enhances HIV infection of Langerhans cells. Cell host & microbe. 2013 Jan 16;13(1):77–86. doi: 10.1016/j.chom.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Chandra N, Thurman AR, Anderson S, et al. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS Research and Human Retroviruses. 2013 Mar;29(3):592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hladik F, Hope TJ. HIV infection of the genital mucosa in women. Curr HIV/AIDS Rep. 2009 Feb;6(1):20–28. doi: 10.1007/s11904-009-0004-1. [DOI] [PubMed] [Google Scholar]
- 44.Introini A, Kaldensjo T, Hirbod T, et al. Expression profiles of antimicrobial peptides in the genital tract of women using progesterone intrauterine devices versus combined oral contraceptives. American journal of reproductive immunology. 2014 Nov;72(5):475–484. doi: 10.1111/aji.12304. [DOI] [PubMed] [Google Scholar]
- 45.King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reproductive biology and endocrinology : RB&E. 2003 Nov 28;1:116. doi: 10.1186/1477-7827-1-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sabatte J, Remes Lenicov F, Cabrini M, et al. The role of semen in sexual transmission of HIV: beyond a carrier for virus particles. Microbes and infection / Institut Pasteur. 2011 Nov;13(12–13):977–982. doi: 10.1016/j.micinf.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Rodriguez-Martinez H, Kvist U, Ernerudh J, Sanz L, Calvete JJ. Seminal plasma proteins: what role do they play? American journal of reproductive immunology. 2011 Jul;66(Suppl 1):11–22. doi: 10.1111/j.1600-0897.2011.01033.x. [DOI] [PubMed] [Google Scholar]
- 48.WHO. Hormonal Contraception and HIV: Technical Statement. Geneva: World Health Organization; [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.