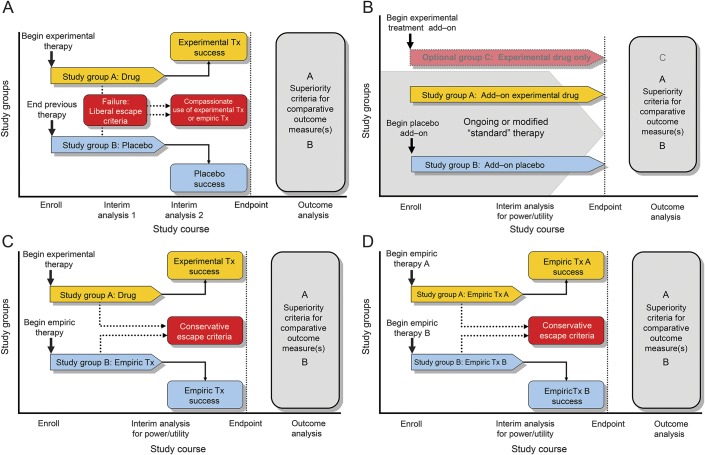
Figure 2. Phase IIb/III study designs for maintenance (attack prevention) treatment.
(A) Patients are randomized to receive experimental treatment (Tx) or placebo. To minimize risk, liberal “escape” criteria are included in the protocol to address on-study disease activity, such as occurrence of first attack. Those whose experimental therapy failed could be reassigned to empiric treatment or an alternate treatment. (B) Patients are maintained on their existing regimen and randomized to receive experimental treatment or placebo as add-on therapy. Inclusion of an optional experimental treatment alone arm (group C) might allow evaluation of potential interaction(s) between empiric and experimental therapy. (C) Patients are randomized to receive either experimental or empiric treatment. (D) Patients are randomized to receive 1 of 2 existing empiric therapies. This design is similar to design C, but 2 empiric treatments are compared directly. For all studies, interim analysis for futility and for assessment of event rate and adjustment of sample size may be conducted with appropriate power correction.