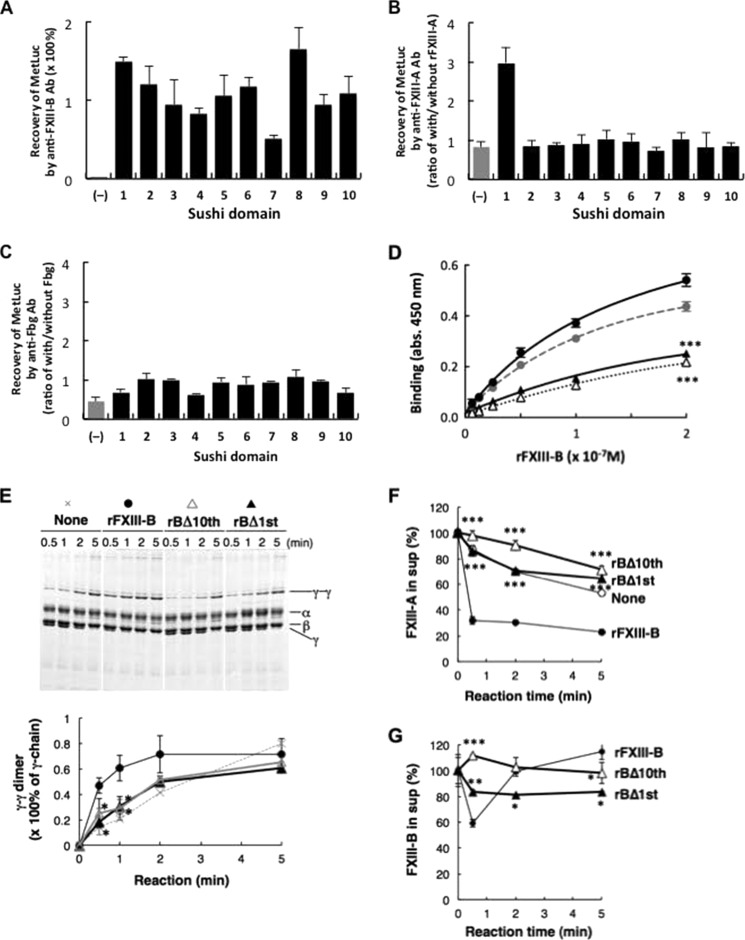
FIGURE 5.
Determination of the Fbg-binding domain in FXIII-B. A–C, binding of MetLuc fused with each sushi domain of FXIII-B to anti-FXIII-B antibody (A), FXIII-A (B), and Fbg (C). Recovery of MetLuc activity collected with anti-FXIII-B-Sepharose is given as a percentage of starting MetLuc preparation (A), and MetLuc activity collected by anti-FXIII-A antibody (B) or anti-Fbg antibody (C) is given as ratio of co-immunoprecipitation with and without rFXIII-A or Fbg, respectively. (−), wild-type MetLuc (without FXIII-B sushi domain). The mean ± S.D. (error bars) of three independent immunoprecipitations is shown. D, kinetic analysis of Fbg binding to rFXIII-B (filled circle), rFXIII-B*3 (gray circle), rBΔ10th (open triangle), and rBΔ1st (filled triangle). Each set of three reactions was plotted. E, effect of truncated rFXIII-B on Fbn cross-linking in FXIII-B(−)+A plasma. The Fbn cross-linking reaction was performed in FXIII-B(−)+A plasma in the presence of rFXIII-B, rBΔ10th, or rBΔ1st, and the Fbn clot was analyzed by SDS-PAGE stained with Coomassie Brilliant Blue dye. Densitometric analyses of γ-γ dimer formation in three independent experiments were performed. Filled circle, rFXIII-B; open triangle, rBΔ10th; filled triangle, rBΔ1st. F and G, effect of truncated FXIII-B constructs on incorporation of FXIII in Fbn clots in human FXIII-B(−)+A plasma in the absence (open circle) or the presence of 5 μg/ml rFXIII-B (filled circle), rBΔ10th (open triangle), or rBΔ1st (filled triangle); FXIII-A (F) and FXIII-B (G) remaining in the supernatant were measured by ELISA. The mean ± S.D. of three reactions is shown. *, p < 0.05; **, p < 0.01; ***, p < 0.001.