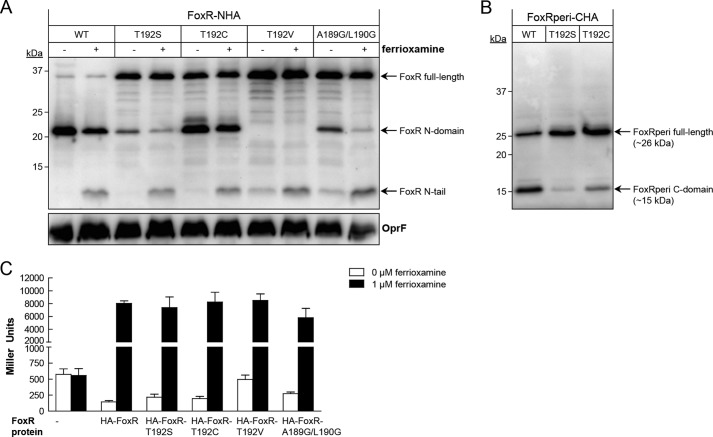
FIGURE 5.
Potential role of an N-O acyl rearrangement in PaFoxR self-cleavage. A, anti-HA tag immunoblot of P. aeruginosa pvdF ΔfoxR carrying the pMMB/HA-FoxR (WT), pMMB/HA-FoxR-T192S, pMMB/HA-FoxR-T192C, pMMB/HA-FoxR-T192V, or pMMB/HA-FoxR-A189G/L190G plasmid. Bacteria were grown under iron-restricted conditions in the presence of 1 mm IPTG without (−) and with (+) 1 μm ferrioxamine. B, Western blot of FoxRperi-CHA incorporating either the T192S or T192V mutation during expression in the PURExpress® system. Proteins were detected using a monoclonal anti-HA tag antibody. The positions of the molecular size marker (in kDa) and the FoxR protein fragments in A and B are indicated. As a loading control in A, a monoclonal antibody against the OprF protein was used. C, β-galactosidase activity of the P. aeruginosa PAO1 pvdF ΔfoxR mutant bearing the pMPR8b plasmid (foxA::lacZ transcriptional fusion) and the pMMB67EH (empty), pMMB/HA-FoxR (WT), pMMB/HA-FoxR-T192S, pMMB/HA-FoxR-T192C, pMMB/HA-FoxR-T192V, or pMMB/HA-FoxR-A189G/L190G plasmid. Bacteria were grown in iron-restricted medium in the absence or presence of 1 μm ferrioxamine.