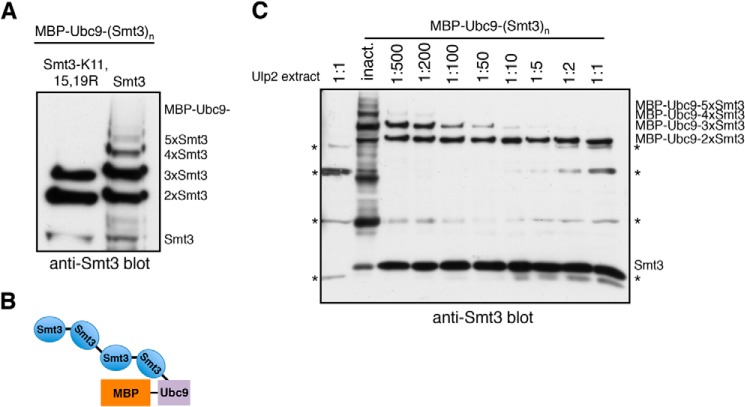
FIGURE 4.
Ulp2 dismantles authentic K-linked poly-Smt3 chains from their distal ends. A, MBP-Ubc9 conjugated to Smt3 was produced in an E. coli-based in vivo sumoylation system. Isopeptide bonds in larger Smt3 chains are formed mostly via the canonical acceptor lysines Lys-11,15,19. B, schematic representation of test substrate obtained from reconstituted sumoylation system in E. coli (in this example: MBP-Ubc9–4xSmt3). C, MBP-Ubc9 conjugated to poly-Smt3 chains of different lengths was incubated with E. coli lysate containing Ulp2 diluted in activity test buffer (1:1 = undiluted lysate). As a control, a lysate was used that contained the inactive C624A variant of Ulp2. Substrates were incubated with different dilutions of Ulp2-containing lysates for 2 h at 30 °C. Reaction products were then analyzed by SDS-PAGE and anti-Smt3 Western blotting. Lysate-derived signals are indicated by the asterisks (*).