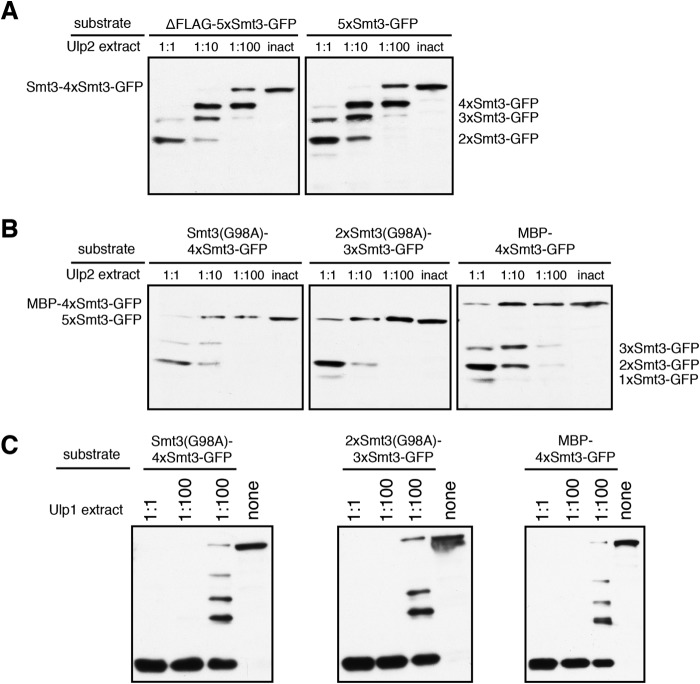
FIGURE 8.
Blocking access to the N terminus at the distal end of a Smt3 chain interferes with cleavage by Ulp2. The indicated substrates were incubated with E. coli lysates containing MBP-Ulp2-FLAG diluted in activity test buffer for 2 h at 30 °C. An undiluted lysate containing the inactive variant (inact.) Ulp2(C624A) was used as a control. Reaction products were analyzed by SDS-PAGE and anti-HA Western blotting. A, comparison of FLAG-tagged (5xSmt3-GFP) and untagged (ΔFLAG-5xSmt3-GFP) substrate variants in a Ulp2 cleavage assay. B, assessing the impact of uncleavable extensions at the distal ends of Smt3 chains on processing by Ulp2. The first two substrates harbor the uncleavable Smt3(G98A) variant in the first or the first two positions. The third substrate carries MBP at the distal end of the chain. C, as in B but using E. coli lysates containing Ulp1 instead of Ulp2. none = no lysate in reaction mix, instead more activity test buffer was used.