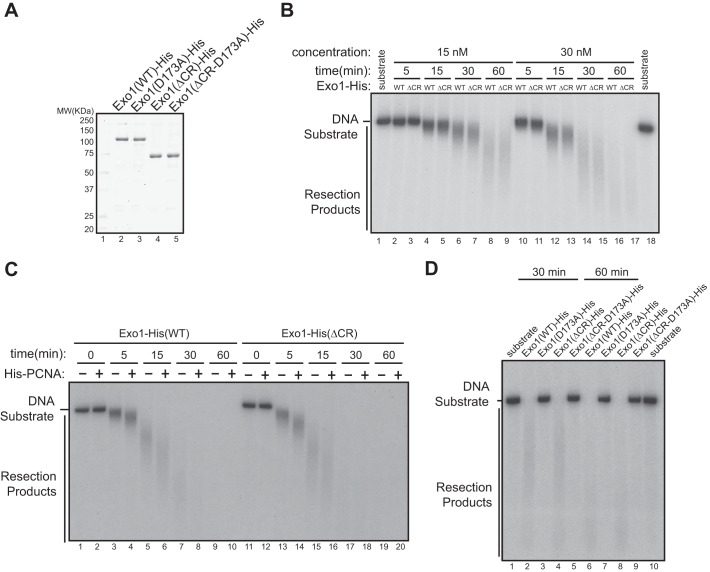
FIGURE 4.
Interaction of 14-3-3s with the central domain of Exo1 does not affect the exonuclease activity of Exo1 in vitro. A, recombinant Exo1(WT)-His, Exo1(D173A)-His, Exo1(ΔCR)-His, and Exo1(ΔCR-D173A)-His proteins were purified from overexpressing Sf9 cells. MW, molecular weight. B, comparison of the exonuclease activities of purified recombinant Exo1(WT)-His and Exo1(ΔCR)-His in vitro toward a 3′ 32P-labeled, 6-kb dsDNA fragment. The reactions included 30 nm of Exo1(WT)-His or Exo1(ΔCR)-His incubated with 2 ng/μl of the DNA substrate. C, PCNA stimulated the resection activity of Exo1(WT)-His and Exo1(ΔCR)-His to a similar extent in vitro using the same reaction conditions as in B. D, in vitro DNA resection activities of Exo1(WT)-His and the mutant proteins shown under the reaction conditions described in B.