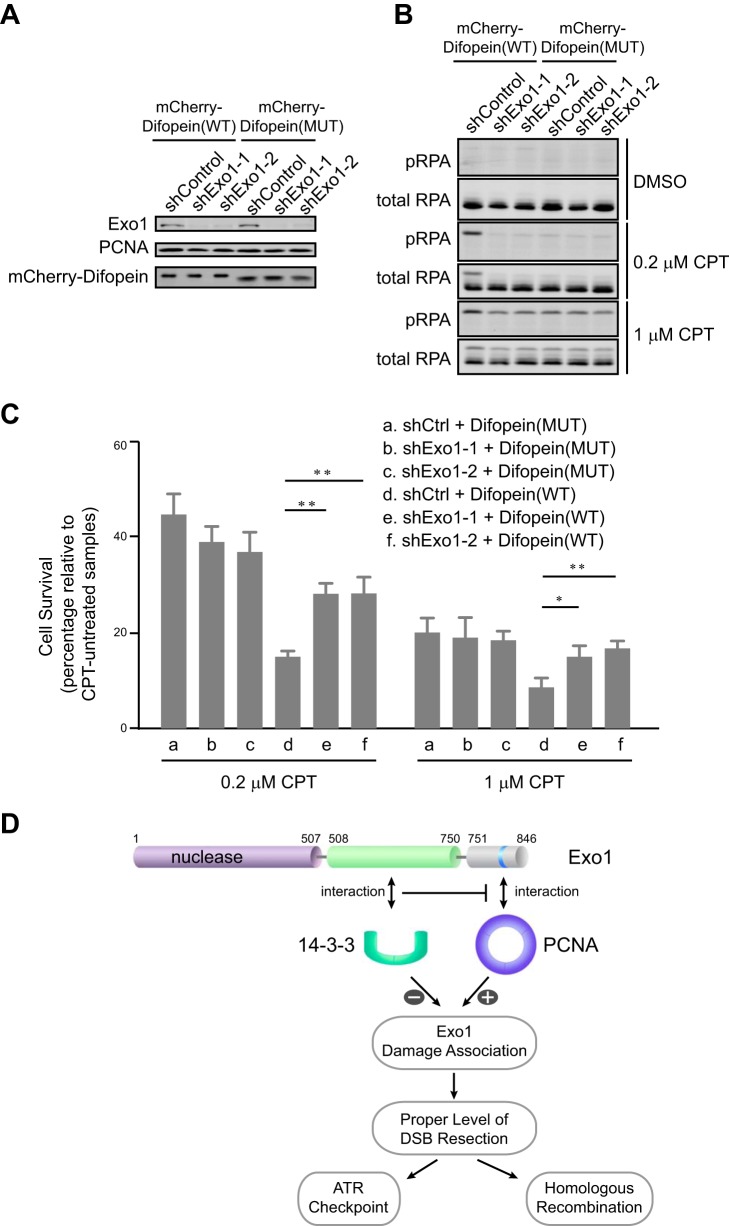
FIGURE 7.
The interaction of Exo1 with 14-3-3s promotes cell survival after DNA damage. A, protein levels of Exo1, mCherry-Difopein, and PCNA in control knockdown or Exo1 knockdown U2OS cells expressing mCherry-Difopein(WT) or mCherry-Difopein(MUT). B, RPA phosphorylation at Ser-4/Ser-8 in control knockdown or Exo1 knockdown cells expressing either mCherry-Difopein(WT) or mCherry-Difopein(MUT) after treatment with dimethyl sulfoxide (DMSO), 0.2 μm camptothecin (CPT), or 1 μm CPT for 3 h, followed by a 3-h recovery period. C, results of a clonogenic survival assay for control knockdown and Exo1 knockdown cells expressing either mCherry-Difopein(WT) or mCherry-Difopein(MUT). Cells were treated with 1 or 0.2 μm camptothecin for 3 h, followed by a 3-h recovery period. Data represent the mean ± S.D. of three independent experiments. *, p < 0.05; **, p < 0.01 (paired Student's t test). D, a model for the regulation of Exo1 in DNA end resection. The association of Exo1 with DNA damage is controlled by Exo1 protein-protein interactions with PCNA and 14-3-3 proteins. PCNA associates with the PCNA-interacting protein box in the C-terminal domain of Exo1 to promote retention at sites of DNA damage and processive resection of DNA breaks. 14-3-3 proteins interact with the central domain of Exo1 and suppress the binding of PCNA to Exo1, thereby limiting the association of Exo1 with DNA damage. The coordinate regulation of Exo1 by the opposing activities of PCNA and 14-3-3s ensures a proper level of DSB resection by Exo1.