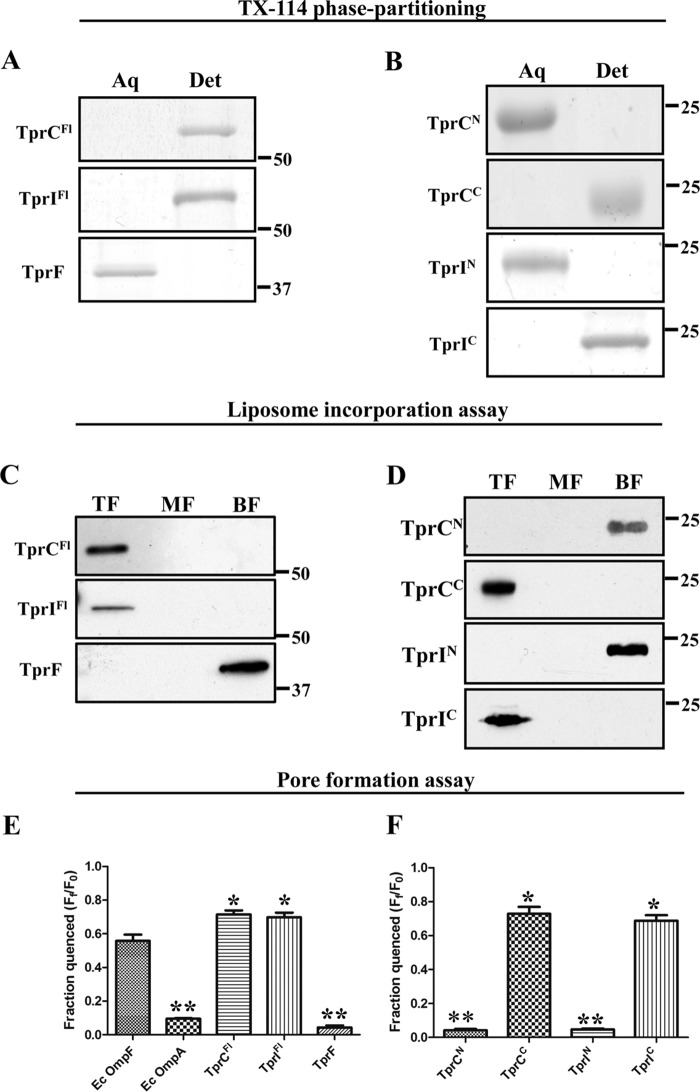
FIGURE 4.
TrpC and TprI, but not TprF, are amphiphilic, pore-forming proteins. A and B, 10 μg of each recombinant protein were phase-partitioned in Triton X-114, separated by SDS-PAGE, and stained with GelCode® Blue. Lanes show aqueous (Aq) and detergent-enriched (Det) phases. C and D, liposomes were reconstituted with 10 μg of each folded recombinant protein, followed by sucrose density gradient ultracentrifugation. Fractions were subjected to SDS-PAGE followed by immunoblotting with antisera directed against TprCFl (TprC and TprI), TprCN (TprCN, TprIN, and TprF), and TprCC (TprCC and TprIC). Lanes show that top fractions (TF) contain liposome-incorporated material, whereas the middle and bottom fractions (MF and BF, respectively) contain unincorporated material. E and F, quenching of LUVs encapsulating Tb(DPA)33− after incubation with 100 nm recombinant proteins in 50 mm HEPES (pH 7.5), 100 mm NaCl supplemented with 5 mm EDTA. Each bar represents the mean ± S.E. (error bars) for three independent experiments. Statistical significance compared with E. coli OmpF was assigned according to the following scheme: *, p < 0.05; **, p < 0.0001. In A–D, molecular mass markers are shown at the right side of each gel or immunoblot.