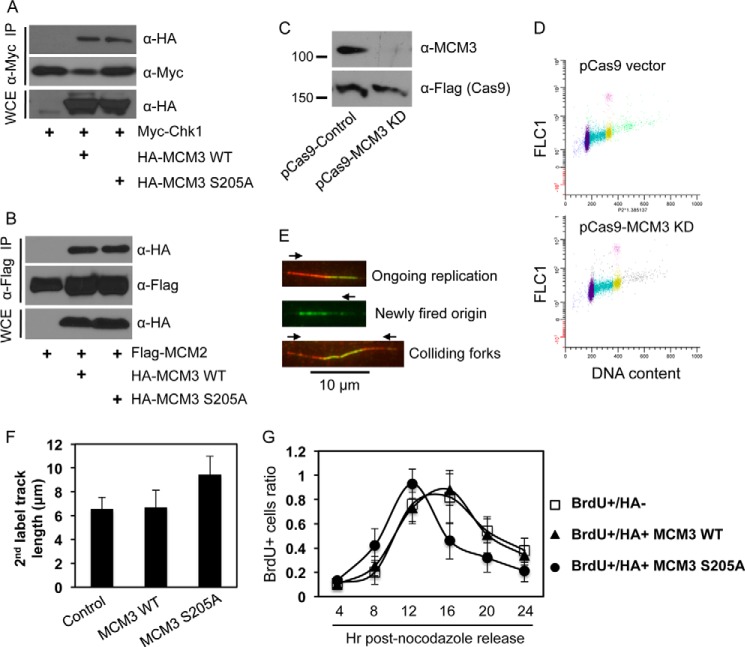
FIGURE 5.
Ser-205 phosphorylation of MCM3 negatively regulates DNA replication and cell cycle progression. A, HEK293T cells were transfected with Myc-Chk1 and HA-MCM3 WT or S205A, IPed with anti-Myc antibodies, and blotted with anti-HA antibodies. The same membrane was stripped and reblotted with anti-Myc antibodies. B, HEK293T cells were transfected with FLAG-MCM2 and HA-MCM3 WT or S205A, IPed with anti-FLAG antibodies, and blotted with anti-HA antibodies followed by stripping and reprobing with anti-FLAG antibodies. C, U2-OS cells were transfected twice in consecutive days with the CRISPR/Cas9 system that targets the 3′- and 5′-UTRs of human MCM3. 48 h after the second transfection (total 72 h of knockdown), cells were collected and immunoblotted with the indicated antibodies. The Cas9 is expressed as a FLAG fusion protein. D, cell cycle profile of U2-OS cells after 72 h of transfection with the control pCas9 or pCas9-MCM3 knockdown (KD) vector. E, DNA fiber assay. U2-OS cells were transfected twice in consecutive days with the CRISPR/Cas9 vectors targeting both the 3′- and 5′-UTRs of MCM3. During the second transfection, HA-MCM3 WT or S205A vectors were also transfected. 48 h after the second transfection, DNA replication in cells was analyzed by the DNA fiber assay as detailed under “Experimental Procedures.” Red and green represent initial and subsequent replication tracks, respectively, taken under a fluorescence microscope (100× oil lens). F, a DNA fiber assay was carried out in U2-OS cells expressing HA-MCM3 WT or S205A mutant under the background of depletion of endogenous MCM3 by the CRISPR/Cas system. The length of actively replicating DNA tracks was analyzed using NIH ImageJ software and adjusted to a standard scale bar of 10 μm taken under the same condition. A minimum of 100 tracks was measured from duplicated experiments, and the experiment was repeated twice. Data represent median and S.D. (error bars). G, U2-OS cells grown on glass coverslips were co-transfected with the CRISPR/Cas MCM3 knockdown plasmid and HA-MCM3 WT or S205A for 72 h, blocked at the G2/M phase by treatment with 200 ng/ml nocodazole for 20 h, and released into the cell cycle. Cells were pulsed with 40 μm BrdU for 15 min at 4, 8, 12, 16, 20, and 24 h after nocodazole release; fixed; immunostained with antibodies against HA and BrdU; and visualized under a fluorescence microscope. The percentage of BrdU-positive cells in HA-negative or HA-positive populations was determined. Data represent median and S.D. (error bars) from duplicate experiments repeated twice. More than 50 cells were counted in each duplicate. WCE, whole cell extracts.