Fig. 3.
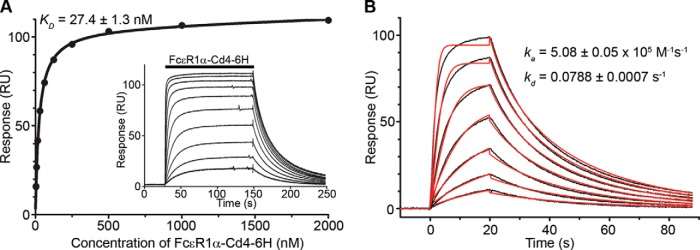
FcεR1α and PEAR1 directly and specifically interact with a relatively high affinity. A, purified monomeric FcεR1α-Cd4-His6 was serially diluted and injected over immobilized PEAR1 until equilibrium was achieved (inset). Binding data that had been reference-subtracted were plotted as a binding curve, and a KD of 27.4 ± 1.3 nm was calculated. B, association and dissociation rate constants derived from an independent kinetic analysis of the FcεR1α-PEAR1 interaction were consistent with the equilibrium analysis. Seven serial dilutions of purified, soluble FcεR1α-Cd4-His6 were injected over immobilized PEAR1 (black lines), and kinetic parameters for the interaction derived from a 1:1 binding model were fitted to the family of sensorgrams (red lines). 6H, His6; RU, response units.
