Abstract
Vertebrate embryos develop in the presence of maternally derived steroids. While these steroids can influence development, embryonic enzymes are thought to buffer some steroid sensitive processes, such as gonadal differentiation, from the effects of maternal steroids. Many of these same enzymes may also buffer the embryo from chemicals present in the environment, but this may alter their capacity to metabolize maternal steroids. Here, we characterized the ability of red-eared slider (Trachemys scripta) embryos to metabolize oestrone immediately following oviposition and tested whether a prevalent environmental chemical, Bisphenol A (BPA), would affect the in ovo conversion of oestrone to oestrone sulfate. We found that tritiated oestrone applied at the time of oviposition is mostly converted to oestrone sulfate within 6 h. However, when BPA is present, that conversion is inhibited, resulting in elevated oestrone levels. Our finding of rapid in ovo metabolism of steroids suggests that maternally derived enzymes are present in the egg and can alter embryonic exposure to exogenous chemicals. The disruption of this metabolism by BPA demonstrates how environmental chemicals might change embryonic exposure to endogenous substances within the egg. Taken together, these findings highlight the dynamic nature of the early endocrine environment in developing vertebrates.
Keywords: oestrone, maternal effect, sulfonation, endocrine disruption
1. Introduction
For most vertebrate species, steroid signals produced by the embryonic gonads during development influence sexual differentiation [1], but intriguingly, this process appears to be largely unaffected by the relatively high levels of steroids in the maternal circulation (placental vertebrates) or egg yolk (oviparous vertebrates) [2]. It appears that maternal steroids are rapidly and efficiently metabolized, effectively ‘buffering’ the embryo from any potential sex-related effects [3–8]. In placental species, tissues such as the placenta and liver possess a suite of enzymes capable of metabolizing steroids as they pass from the maternal to the fetal circulation [9]. Recently, it has been shown that maternal steroids are also metabolized by embryos of oviparous vertebrates [6,7,10,11]. While the metabolic fate of steroids can vary depending on a number of factors including the steroid of interest, study species and period of embryonic development, the general pattern is for steroids to be subject to Phase I metabolism (i.e. oxidation or reduction) [12] followed by Phase II metabolism (i.e. sulfonation or glucuronidation) which is typically associated with inactivation and clearance [13].
In all vertebrate embryos, the Phase II metabolism of maternal steroids is primarily accomplished by sulfonation [8,14], replaced with glucuronidation after birth [15]. Much of what we know about the sulfonation of maternal steroids in oviparous amniotes comes from work done in the red-eared slider (Trachemys scripta). Research initially focused on the metabolic fate of maternal oestradiol because exogenous oestradiol can influence gonadal differentiation [16], and because levels of maternally derived oestradiol in eggs vary seasonally; second clutches contain approximately 10× more oestradiol than first clutches from the same female within a nesting season [17]. During the first 10 days of incubation (approx. 15–25% of total incubation depending upon incubation temperature), concentrations of oestradiol in the yolk drop to low/undetectable levels regardless of initial concentrations [17]. This decline is driven by the conversion of oestradiol to oestrone sulfate, resulting in increased oestrone sulfate levels early in development [8]. Exogenous oestrone sulfate can cause gonadal feminization, but only at very high physiological or supraphysiological levels [8]. Consequently, sulfonation has been hypothesized to buffer the developing embryo from the effects of maternal oestrogens in a similar manner to what is observed in placental amniotes [6,8,18–20].
Many of the same sulfotransferase enzymes that are responsible for the sulfonation of steroids also sulfonate exogenous chemicals including endocrine disrupting chemicals (EDCs) [20]. Thus, maternally derived steroids and EDCs could both be conjugated by the same enzymes during embryonic development. We have recently demonstrated that the application of the EDC, Bisphenol A (BPA), to T. scripta eggs results in increased levels of oestradiol and oestrone and decreased levels of oestrone sulfate present within the egg during early development [21]. In this study, we test the hypothesis that BPA inhibits the conversion of oestrone to oestrone sulfate. Because the sulfonation of exogenous oestrogens occurs very early in development for T. scripta, we first characterize the sulfonation of oestrone over the first 72 h of incubation, and then examine the effect of BPA on the conversion of oestrone to oestrone sulfate.
2. Material and methods
To characterize the time course of oestrone sulfonation, five clutches of T. scripta eggs were collected from gravid females inhabiting Banner Marsh State Fish and Wildlife Area (Fulton Co., IL, USA) during the summer of 2012. Within 24 h of oviposition, nine eggs from each clutch were topically dosed with 150 000 cpm of [2,4,6,7-3H] oestrone (NET319; Perkin Elmer, Boston, MA, USA) dissolved in 5 µl of 70% ethanol. Eggs were then incubated at 31°C and one egg per clutch was frozen at 0.08, 0.5, 1, 3, 6, 12, 24, 48 and 72 h following treatment.
The effect of BPA on the sulfonation of oestrone was characterized in a similar manner using five additional clutches of T. scripta eggs. For this study, clutches were divided into two treatments. Half of the eggs (Control) were dosed with 150 000 cpm of [2,4,6,7-3H] oestrone dissolved in 5 µl of 70% ethanol. The other half (BPA-treated) were dosed with 150 000 cpm of [2,4,6,7-3H] oestrone plus 40 µg of BPA dissolved in 5 µl of 70% ethanol. Eggs were then incubated at 31°C and one egg per clutch/treatment was frozen at 12, 24, 48, 72 and 96 h following treatment. Eggs were sampled by removing the shell from frozen eggs and homogenizing all internal egg components (albumen, yolk and embryo). Details on how the distribution of radioactivity was characterized and analysed can be found in the electronic supplementary material.
3. Results
Levels of ether-soluble radioactivity (oestrone) decreased very rapidly following application to the eggshell (F8,47 = 30.69, p < 0.0001) (figure 1a), and this decline occurs in conjunction with a rapid increase in water-soluble radioactivity inside the egg (oestrone sulfate) (F8,47 = 130.13, p < 0.0001) (figure 1b). TLC analysis indicated that only oestrone was present in the ether-soluble fraction and only oestrone sulfate was present in the water-soluble fraction as secondary peaks were not observed in any samples (electronic supplementary material, figure S1). For the time course study, an average of 79% of the applied radioactivity was recovered.
Figure 1.
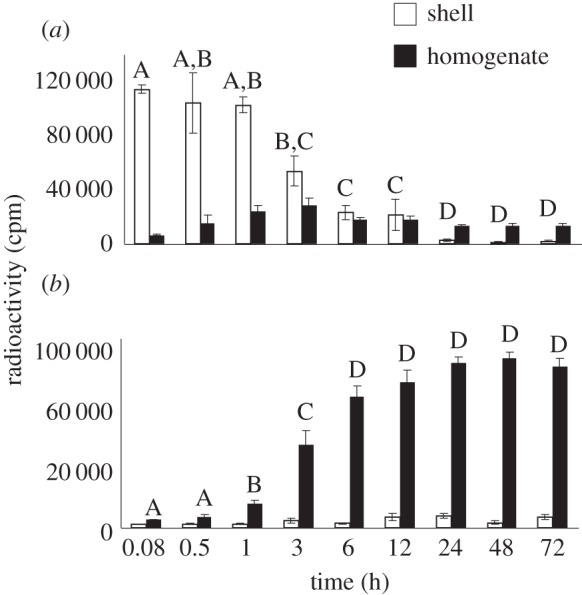
Distribution of ether-soluble (a) and water-soluble (b) radioactivity during the first 72 h of development following exogenous application of [3H]-oestrone to the egg shell at oviposition.
BPA had a strong effect on the conversion of oestrone to oestrone sulfate. In BPA-treated eggs, levels of ether-soluble radioactivity (oestrone) within the homogenate were significantly elevated (F9,41 = 9.40, p < 0.0001) (figure 2a), while levels of water-soluble radioactivity (oestrone sulfate) were significantly lower (F9,41 = 56.15, p < 0.0001) (figure 2b). As with the first study, TLC analysis indicated that only oestrone was present in the ether-soluble fraction and only oestrone sulfate was present in the water-soluble fraction [8,19]. For this study, an average of 62% of the applied radioactivity was recovered.
Figure 2.
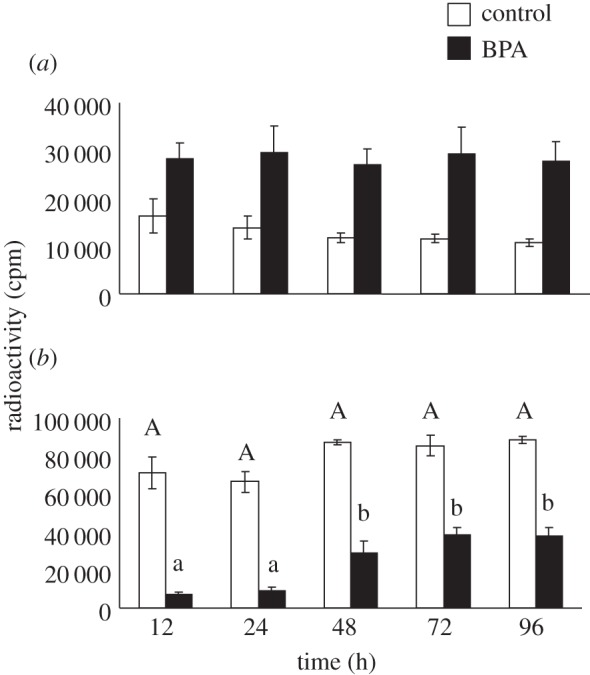
The effect of Bisphenol A on the conversion of oestrone ((a) ether-soluble) to oestrone sulfate ((b) water-soluble) during the first 96 h of development.
4. Discussion
Two key findings come from these studies. First, we found that topically applied oestrone is essentially absent from the shell within about 24 h of application (figure 1a). This decline is not simply due to oestrone moving into the egg, but to oestrone being converted to oestrone sulfate upon entering the egg as evidenced by the lack of ether-soluble oestrone within the egg at any point in development (figure 1a). Second, the sulfonation of oestrone is subject to inhibition by a common environmental chemical, BPA. Relative to control eggs, BPA-treated eggs had increased levels of oestrone and decreased levels of oestrone sulfate, which could result in inappropriate embryonic exposure to endogenous steroids. These findings have important implications for understanding how maternal steroids and EDCs might act during development.
Maternally derived steroidogenic enzymes are present in unfertilized amphibian [22] eggs, but have not been previously documented in amniote eggs. Here, we demonstrate rapid in ovo metabolism of oestrone in T. scripta, providing evidence of maternally derived steroidogenic enzyme activity in an amniote egg. Although T. scripta eggs clearly contain steroidogenic enzymes at oviposition, it is unclear whether these same enzymes are responsible for the in ovo metabolism of maternally derived steroids in the yolk. We have previously reported that maternally derived progesterone, testosterone and oestradiol are all metabolized [8], but that concentrations do not decline to undetectable levels until the tenth day of development [17], while in the current study, exogenous oestrone was metabolized to undetectable levels within hours. Initially, steroidogenic enzymes may only be present in the periphery of the egg, not coming into contact with endogenous steroids that are primarily located within the yolk until later in development, when the yolk migrates from the centre towards the top of the egg during early development [23]. Alternatively, embryonically produced enzymes may be present by day 10 and metabolize steroids in the yolk, with maternal enzymes primarily functioning to buffer the embryo from external compounds. However, we feel it is likely that maternal enzymes can influence steroid metabolism in the yolk given that by day 10, minimal embryonic development has taken place. Nonetheless, the presence of maternally derived enzymes in eggs makes it possible for exogenous substances to be metabolized as they are absorbed into the egg and affects how we should interpret outcomes of studies that use exogenous steroids or ‘steroid-like’ substances such as EDCs.
The capacity to metabolize substances as they enter the egg may represent a powerful buffer such as the ‘chemical defensome’ that has been described in species that lay eggs in aquatic environments [24]. Regardless of whether or not these enzymes serve to defend embryos from environmental chemicals, such as BPA, data from the current study exemplify just how dynamic exposure to exogenous chemicals can be. In control eggs, exogenous oestrone was essentially eliminated from the egg within 6 h, being converted to oestrone sulfate, highlighting the fact that the applied substance is not necessarily what reaches the embryo. In BPA-treated eggs, the presence of BPA increased the amount of oestrone present in the egg, highlighting the fact that embryonic exposure to maternal steroids can be altered by exposure to substances such as EDCs. Ultimately more work is needed to decipher the role of maternally derived enzymes in the in ovo metabolism of maternally derived steroids and environmental chemicals.
Supplementary Material
Supplementary Material
Supplementary Material
Acknowledgements
We thank Pat Wagner for his assistance with this project.
Ethics statement
All experiments were performed under permission of the Illinois State University Institutional Animal Care and Use Committee (IACUC protocol 04–2010) and the Illinois Department of Natural Resources (IDNR permit NH12.2084).
Data accessibility
Datasets supporting this article can be found in the electronic supplementary material.
Funding statement
This research was supported by NSF grant no. IOS-0952840 and NIH grant no. R15ES023995.
Authors' contributions
R.T.P. and R.M.B. designed the study. R.T.P. conducted the study and analysed the data. R.T.P. and R.M.B. wrote the manuscript.
Conflict of interests
We have no competing interests.
References
- 1.Gerall AA, Moltz H, Ward IL. 1992. Handbook of behavioral neurobiology, vol. 11. Sexual differentiation. New York, NY: Plenum. [Google Scholar]
- 2.Carere C, Balthazart J. 2007. Sexual versus individual differentiation: the controversial role of avian maternal hormones. Trends Endocrinol. Metab. 18, 73–80. ( 10.1016/j.tem.2007.01.003) [DOI] [PubMed] [Google Scholar]
- 3.Diczfalusy E. 1963. Endocrine functions of the human fetoplacental unit. Am. J. Obstet. Gynecol. 23, 791–798. ( 10.1016/j.ajog.2005.02.117) [DOI] [PubMed] [Google Scholar]
- 4.Levitz M. 1966. Conjugation and transfer of fetal–placental steroid hormones. J. Clin. Endocrinol. Metab. 26, 773–777. ( 10.1210/jcem-26-7-773) [DOI] [PubMed] [Google Scholar]
- 5.Painter DL, Moore MC. 2005. Steroid hormone metabolism by the chorioallantoic placenta of the mountain spiny lizard (Sceloporus jarrovi) as a possible mechanism for buffering maternal–fetal hormone exchange. Physiol. Biochem. Zool. 78, 364–372. ( 10.1086/430222) [DOI] [PubMed] [Google Scholar]
- 6.Paitz RT, Bowden RM. 2008. A proposed role of the sulfotransferase/sulfatase pathway in modulating yolk steroid effects. Integr. Comp. Biol. 48, 419–427. ( 10.1093/icb/icn034) [DOI] [PubMed] [Google Scholar]
- 7.von Engelhardt N, Henriksen R, Groothuis TGG. 2009. Steroids in chicken egg yolk: metabolism and uptake during early embryonic development. Gen. Comp. Endocr. 163, 175–183. ( 10.1016/j.ygcen.2009.04.004) [DOI] [PubMed] [Google Scholar]
- 8.Paitz RT, Bowden RM. 2013. Sulfonation of maternal steroids is a conserved metabolic pathway in vertebrates. Integr. Comp. Biol. 53, 895–901. ( 10.1093/icb/ict027) [DOI] [PubMed] [Google Scholar]
- 9.Pasqualini JR. 2005. Enzymes involved in the formation and transformation of steroid hormones in the fetal and placental compartments. J. Steroid Biochem. Mol. Biol. 97, 401–415. ( 10.1016/j.jsbmb.2005.08.004) [DOI] [PubMed] [Google Scholar]
- 10.Paitz RT, Bowden RM, Casto JM. 2011. Embryonic modulation of maternal steroids in European starlings (Sturnus vulgaris). Proc. R. Soc. B 278, 99–106. ( 10.1098/rspb.2010.0813) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vassallo BG, Paitz RT, Fasanello VJ, Haussmann MF. 2014. Glucocorticoid metabolism in the in ovo environment modulates exposure to maternal corticosterone in Japanese quail embryos (Coturnix japonica). Biol. Lett. 10, 20140502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorfman RI, Ungar F. 1965. Metabolism of steroid hormones. New York, NY: Academic Press. [Google Scholar]
- 13.Bernstein S, Solomon S. 1970. Chemical and biological aspects of steroid conjugation. Berlin, Germany: Springer-Verlag. [Google Scholar]
- 14.Diczfalusy E, Levitz M. 1970. Formation, metabolism, and transport of estrogen conjugates. In Chemical and biological aspects of steroid conjugation (eds S Bernstein, S Solomon), pp. 291–320. Berlin, Germany: Springer. [Google Scholar]
- 15.Dutton GJ. 1978. Developmental aspects of drug conjugation, with special reference to glucuronidation. Annu. Rev. Pharmacol. Toxicol. 18, 17–35. ( 10.1146/annurev.pa.18.040178.000313) [DOI] [PubMed] [Google Scholar]
- 16.Wibbels T, Bull JJ, Crew D. 1994. Temperature-dependent sex determination: a mechanistic approach. J. Exp. Zool. 270, 71–78. ( 10.1002/jez.1402700108) [DOI] [PubMed] [Google Scholar]
- 17.Paitz RT, Bowden RM. 2009. Rapid decline in the concentrations of three yolk steroids during development: is it embryonic regulation? Gen. Comp. Endocr. 161, 246–251. ( 10.1016/j.ygcen.2009.01.018) [DOI] [PubMed] [Google Scholar]
- 18.Paitz RT, Bowden RM. 2010. Progesterone metabolites, ‘xenobiotic-sensing’ nuclear receptors, and the metabolism of maternal steroids. Gen. Comp. Endocr. 166, 217–221. ( 10.1016/j.ygcen.2009.11.011) [DOI] [PubMed] [Google Scholar]
- 19.Paitz RT, Sawa AR, Bowden RM. 2012. Characterizing the metabolism and movement of yolk estradiol during embryonic development in the red-eared slider (Trachemys scripta). Gen. Comp. Endocr. 176, 507–512. ( 10.1016/j.ygcen.2011.10.009) [DOI] [PubMed] [Google Scholar]
- 20.Paitz RT, Bowden RM. 2011. Biological activity of oestradiol sulphate in an oviparous amniote: implications for maternal steroid effects. Proc. R. Soc. B 278, 2005–2010. ( 10.1098/rspb.2010.2128) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Clairardin SG, Paitz RT, Bowden RM. 2013. In ovo inhibition of steroid metabolism by Bisphenol-A as a potential mechanism of endocrine disruption. Proc. R. Soc. B 280, 20131773 ( 10.1098/rspb.2013.1773) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fouchet C, Serres C, Bellé R, Ozon R. 1975. Mechanism of action of progesterone on amphibian oocytes. Uptake and metabolism of progesterone by isolated oocytes of Pleurodeles waltlii and Xenopus laevis. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 52, 205–210. ( 10.1016/0305-0491(75)90053-X) [DOI] [PubMed] [Google Scholar]
- 23.Ewert MA. 1985. Embryology of turtles. In Biology of the Reptilia (eds Gans C, Billett FS, Maderson P.), pp. 77–241. New York, NY: Wiley, Inc. [Google Scholar]
- 24.Goldstone JV, Hamdoun A, Cole BJ, Howard-Ashby M, Nebert DW, Scally M, Stegeman JJ. 2006. The chemical defensome: environmental sensing and response genes in the Strongylocentrotus purpuratus genome. Dev. Biol. 300, 366–384. ( 10.1016/j.ydbio.2006.08.066) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Datasets supporting this article can be found in the electronic supplementary material.


