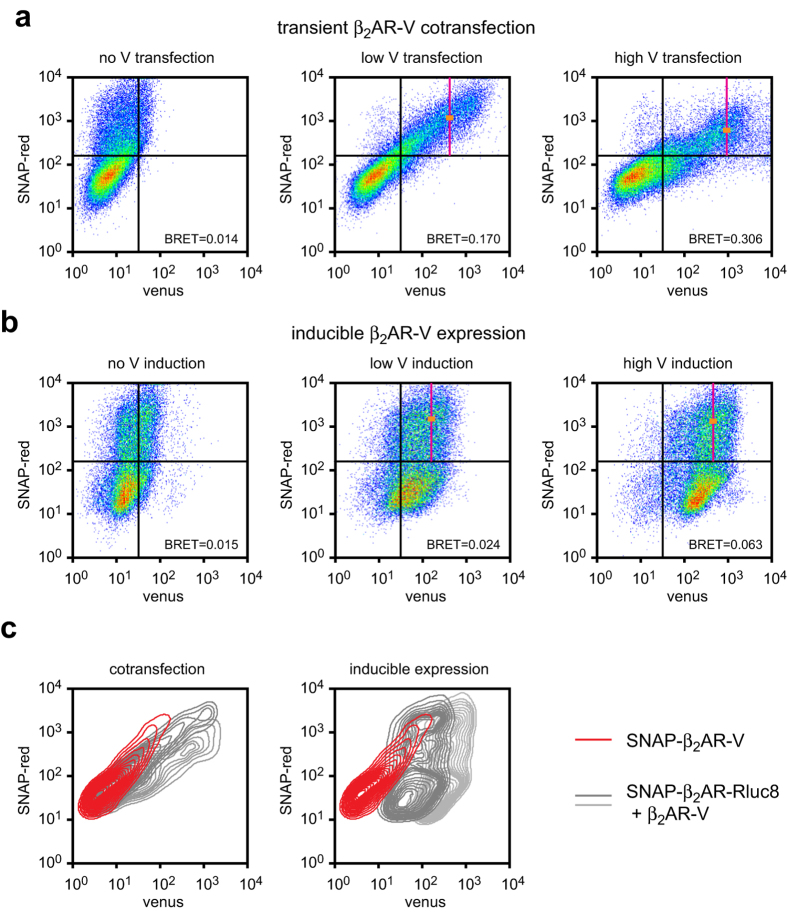
Figure 4. Donor and acceptor expression are positively correlated after transient contransfection.
SNAP-tagged β2AR-Rluc8 (covalently labeled with SNAP-red) and β2AR-V expression were measured by flow cytometry. Gates were defined by staining untransfected cells with SNAP-red. Mean β2AR-V expression in coexpressing (upper right quadrant) cells is indicated by a vertical magenta line, and mean β2AR-Rluc8 expression in the same cells is indicated by an orange tick. BRET measured from each population of cells is indicated in each panel. (a) Transient cotransfection of constant SNAP-β2AR-Rluc8 (0.5 μg well-1) and increasing β2AR-V (0-2.5 μg well−1) produced positively correlated expression of the two proteins. Increasing the amount of β2AR-V DNA from 0.5 μg well−1 to 2.5 μg well−1 increased average β2AR-V expression in coexpressing cells from 426 arbitrary units (a.u.; CV = 1.54) to 936 a.u. (CV = 1.60), and decreased average β2AR-Rluc8 expression from 1,188 a.u. (CV = 1.13) to 612 a.u. (CV = 1.13). (b) Transient transfection of constant SNAP-β2AR-Rluc8 (0.5 μg well−1) with tetracycline induction of β2AR-V produced uncorrelated expression of the two proteins. Increasing the tetracycline concentration increased average β2AR-V expression in coexpressing cells from 162 a.u. (CV = 0.83) to 458 a.u. (CV = 0.82), and decreased average β2AR-Rluc8 expression from 1,502 a.u. (CV = 1.11) to 1,328 a.u. (CV = 1.13). (c) Cells expressing receptors with a single SNAP tag and a single venus (SNAP-β2AR-venus) were stained with SNAP-red and analyzed by flow cytometry (red contour plots). Superimposed (grey) contour plots from cells expressing SNAP-β2AR-Rluc8 and β2AR-V by both methods (the same cells as in panels a and b) show that most cells express more β2AR-V than SNAP-β2AR-Rluc8. This level of SNAP-β2AR-Rluc8 expression (48 hours after transfection) corresponds to the highest level of β2AR-Rluc8 expression in Fig. 1, and produced similar levels of luminescence. Each contour line represents 5% of the total cell population. Data are representative of 3 identical flow cytometry experiments.