Abstract
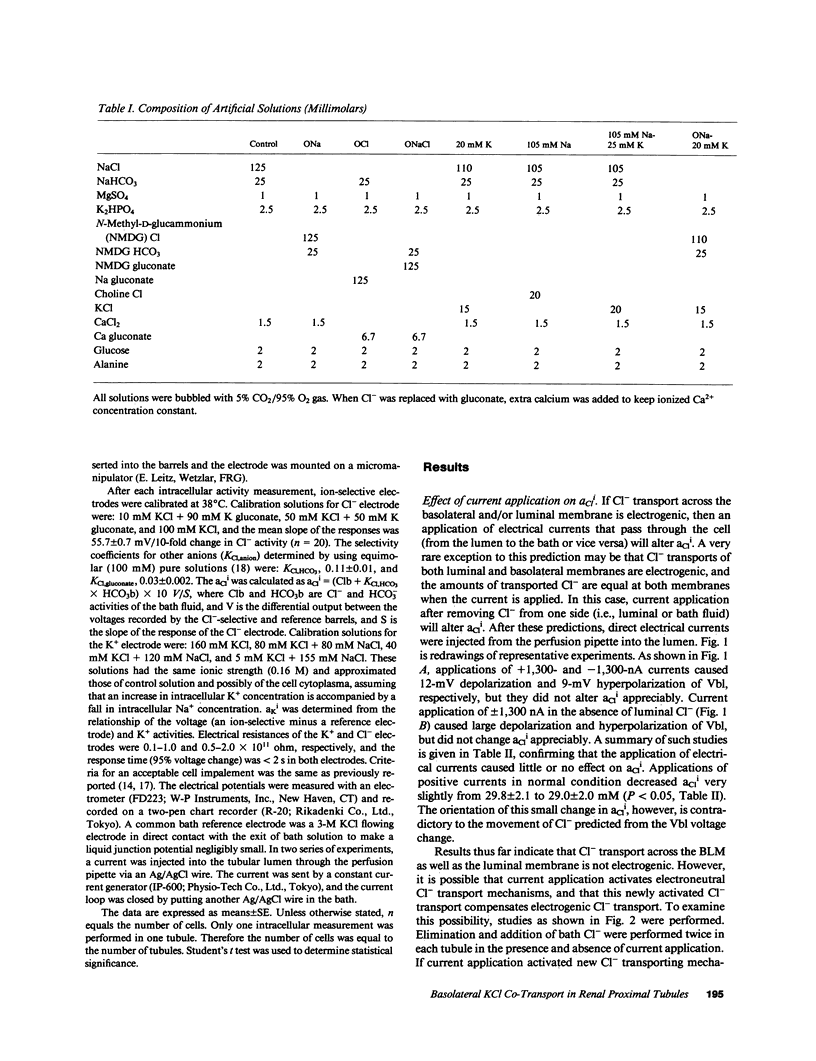
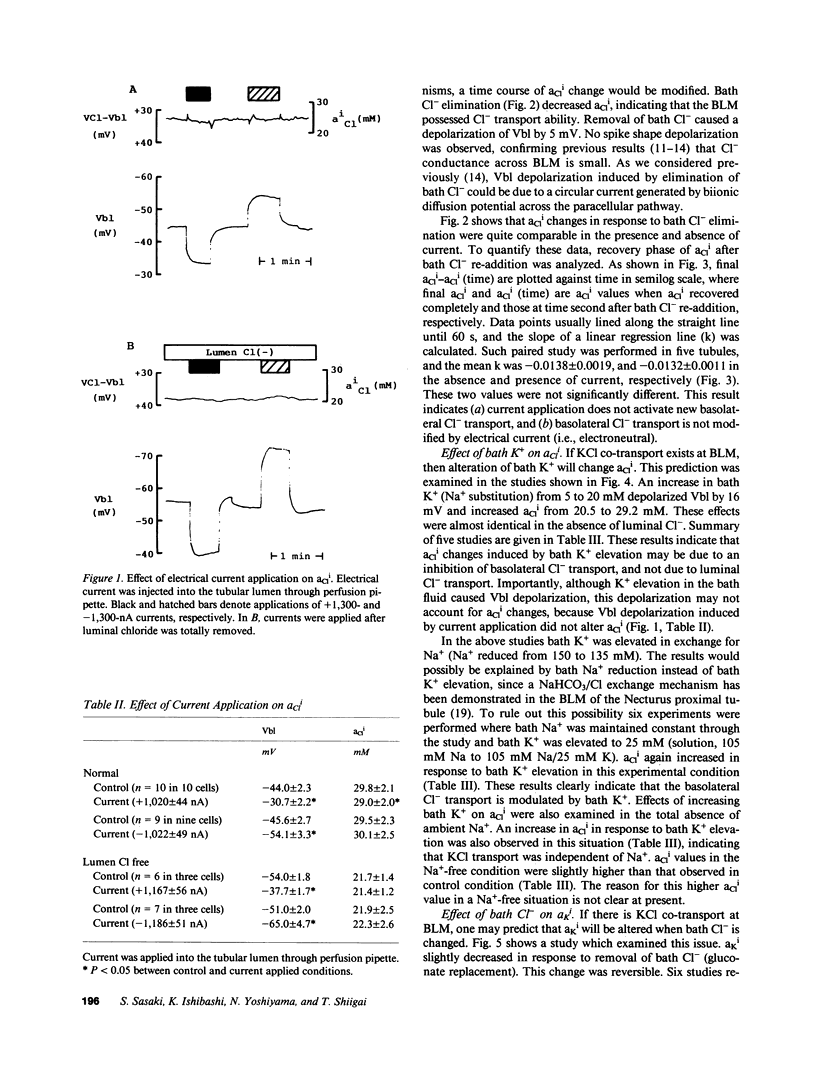
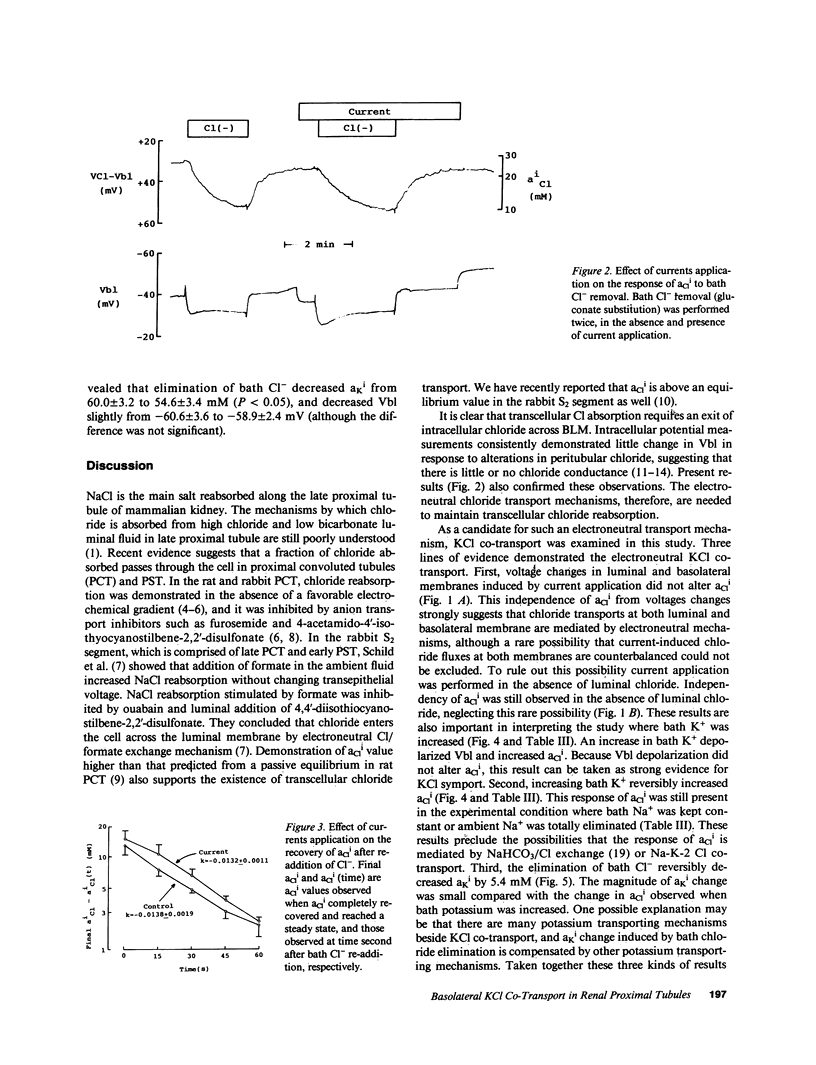
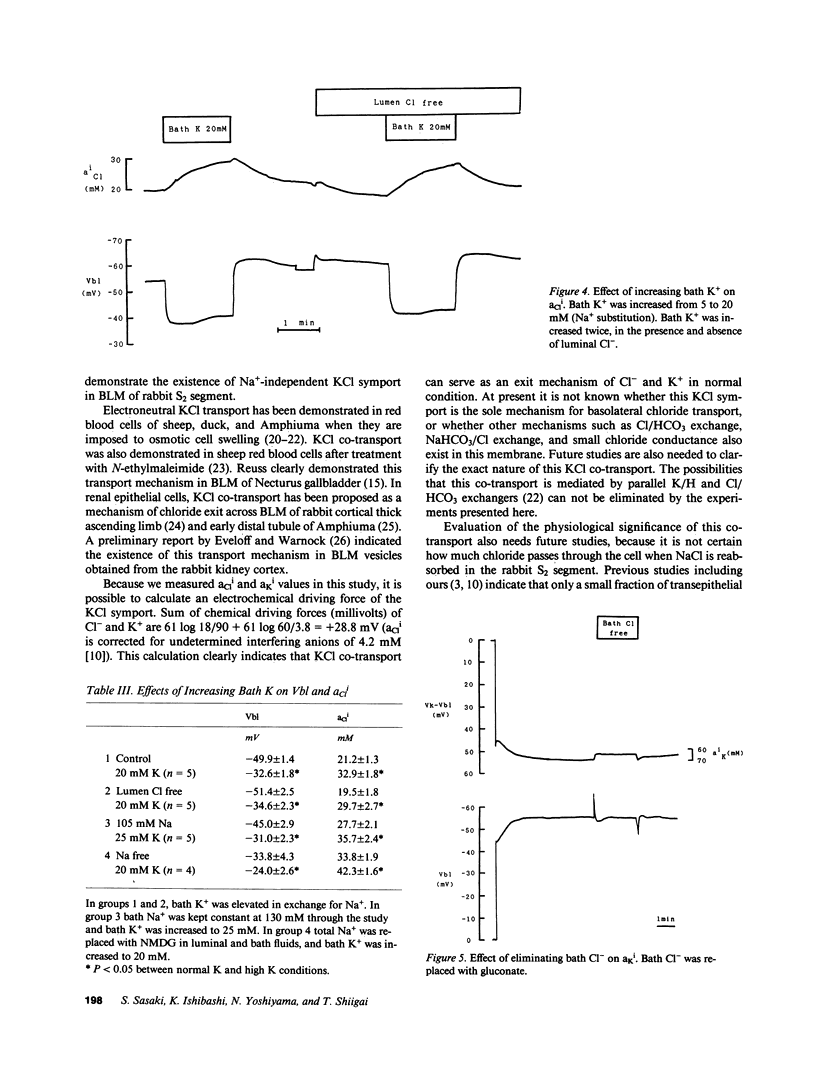
Mammalian renal proximal tubules reabsorb large amounts of chloride. Mechanisms of the transcellular chloride transport are poorly understood. To determine whether KCl co-transport exists in the basolateral membrane of mammalian renal proximal tubule, isolated rabbit proximal straight tubules (S2 segment) were perfused in vitro, and intracellular activities of potassium and chloride (aKi, aCli) were measured by double-barreled ion-selective microelectrodes. aCli did not change when basolateral membrane voltage was altered by application of a direct current through perfusion pipette. aCli changes in response to bath chloride elimination were not affected by current application as well, indicating that the basolateral chloride transport is electroneutral. An increase in potassium concentration of the bath fluid from 5 to 20 mM reversibly increased aCli by 10 mM. This response of aCli to a change in the bath potassium concentration was also observed when luminal chloride was removed, or ambient sodium was totally removed. aKi significantly decreased by 5 mM when chloride was removed from the bath. These data demonstrate the existence of an electroneutral Na+-independent KCl co-transport in the basolateral membrane of the rabbit proximal tubule. Calculated electrochemical driving force was favorable for the movement of KCl from the cell to the peritubular fluid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpern R. J., Howlin K. J., Preisig P. A. Active and passive components of chloride transport in the rat proximal convoluted tubule. J Clin Invest. 1985 Oct;76(4):1360–1366. doi: 10.1172/JCI112111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M., Berry C. A. Evidence for neutral transcellular NaCl transport and neutral basolateral chloride exit in the rabbit proximal convoluted tubule. J Clin Invest. 1984 Jul;74(1):205–211. doi: 10.1172/JCI111403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum M. Evidence that parallel Na+-H+ and Cl(-)-HCO3-(OH-) antiporters transport NaCl in the proximal tubule. Am J Physiol. 1987 Feb;252(2 Pt 2):F338–F345. doi: 10.1152/ajprenal.1987.252.2.F338. [DOI] [PubMed] [Google Scholar]
- Bello-Reuss E. Electrical properties of the basolateral membrane of the straight portion of the rabbit proximal renal tubule. J Physiol. 1982 May;326:49–63. doi: 10.1113/jphysiol.1982.sp014176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burckhardt B. C., Cassola A. C., Frömter E. Electrophysiological analysis of bicarbonate permeation across the peritubular cell membrane of rat kidney proximal tubule. II. Exclusion of HCO3(-)-effects on other ion permeabilities and of coupled electroneutral HCO3(-)-transport. Pflugers Arch. 1984 May;401(1):43–51. doi: 10.1007/BF00581531. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Cala P. M. Volume regulation by Amphiuma red blood cells. The membrane potential and its implications regarding the nature of the ion-flux pathways. J Gen Physiol. 1980 Dec;76(6):683–708. doi: 10.1085/jgp.76.6.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal J., Lapointe J. Y., Laprade R. Luminal and peritubular ionic substitutions and intracellular potential of the rabbit proximal convoluted tubule. Am J Physiol. 1984 Aug;247(2 Pt 2):F352–F364. doi: 10.1152/ajprenal.1984.247.2.F352. [DOI] [PubMed] [Google Scholar]
- Cassola A. C., Mollenhauer M., Frömter E. The intracellular chloride activity of rat kidney proximal tubular cells. Pflugers Arch. 1983 Dec;399(4):259–265. doi: 10.1007/BF00652749. [DOI] [PubMed] [Google Scholar]
- Dunham P. B., Ellory J. C. Passive potassium transport in low potassium sheep red cells: dependence upon cell volume and chloride. J Physiol. 1981 Sep;318:511–530. doi: 10.1113/jphysiol.1981.sp013881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Rumrich G., Ullrich K. J. Phenomenologic description of Na+, Cl- and HCO-3 absorption from proximal tubules of rat kidney. Pflugers Arch. 1973 Oct 22;343(3):189–220. doi: 10.1007/BF00586045. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., Boulpaep E. L., Giebisch G. Electrical properties of chloride transport across the necturus proximal tubule. J Membr Biol. 1982;65(3):185–196. doi: 10.1007/BF01869962. [DOI] [PubMed] [Google Scholar]
- Guggino W. B. Functional heterogeneity in the early distal tubule of the Amphiuma kidney: evidence for two modes of Cl- and K+ transport across the basolateral cell membrane. Am J Physiol. 1986 Mar;250(3 Pt 2):F430–F440. doi: 10.1152/ajprenal.1986.250.3.F430. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., London R., Boulpaep E. L., Giebisch G. Chloride transport across the basolateral cell membrane of the Necturus proximal tubule: dependence on bicarbonate and sodium. J Membr Biol. 1983;71(3):227–240. doi: 10.1007/BF01875464. [DOI] [PubMed] [Google Scholar]
- Kregenow F. M. Osmoregulatory salt transporting mechanisms: control of cell volume in anisotonic media. Annu Rev Physiol. 1981;43:493–505. doi: 10.1146/annurev.ph.43.030181.002425. [DOI] [PubMed] [Google Scholar]
- Lauf P. K. Thiol-dependent passive K/Cl transport in sheep red cells: I. Dependence on chloride and external ions. J Membr Biol. 1983;73(3):237–246. doi: 10.1007/BF01870538. [DOI] [PubMed] [Google Scholar]
- Lucci M. S., Warnock D. G. Effects of anion-transport inhibitors on NaCl reabsorption in the rat superficial proximal convoluted tubule. J Clin Invest. 1979 Aug;64(2):570–579. doi: 10.1172/JCI109495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rector F. C., Jr Sodium, bicarbonate, and chloride absorption by the proximal tubule. Am J Physiol. 1983 May;244(5):F461–F471. doi: 10.1152/ajprenal.1983.244.5.F461. [DOI] [PubMed] [Google Scholar]
- Reuss L. Basolateral KCl co-transport in a NaCl-absorbing epithelium. Nature. 1983 Oct 20;305(5936):723–726. doi: 10.1038/305723a0. [DOI] [PubMed] [Google Scholar]
- Sasaki S., Shigai T., Takeuchi J. Intracellular pH in the isolated perfused rabbit proximal straight tubule. Am J Physiol. 1985 Sep;249(3 Pt 2):F417–F423. doi: 10.1152/ajprenal.1985.249.3.F417. [DOI] [PubMed] [Google Scholar]
- Schafer J. A., Troutman S. L., Watkins M. L., Andreoli T. E. Volume absorption in the pars recta. I. "Simple" active Na+ transport. Am J Physiol. 1978 Apr;234(4):F332–F339. doi: 10.1152/ajprenal.1978.234.4.F332. [DOI] [PubMed] [Google Scholar]
- Schild L., Giebisch G., Karniski L. P., Aronson P. S. Effect of formate on volume reabsorption in the rabbit proximal tubule. J Clin Invest. 1987 Jan;79(1):32–38. doi: 10.1172/JCI112803. [DOI] [PMC free article] [PubMed] [Google Scholar]


