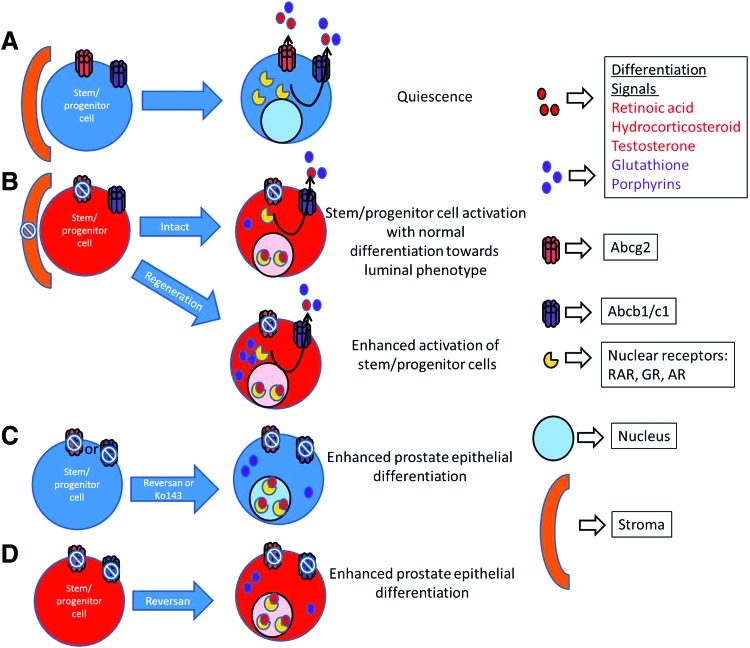
FIG. 6.
Model of enhanced differentiation through MDR-ABC efflux transporter inhibition. (A) Cells with active MDR-ABC efflux transporters are quiescent and have undetectable steroid receptors and differentiation signals. (B) Systemic Abcg2 deletion/inhibition in stroma and epithelia leads to activation of stem/progenitor cell niche and normal differentiation toward luminal phenotype. Moreover, after prostate regression and regeneration cycles, Abcg2 deletion leads to enhanced activation of stem/progenitor cells. Enhanced activation of stem/progenitor niche may be due to oxidative stress induced by androgen deprivation and the highest accumulation of glutathione and porphyrins. (C) Complete MDR-ABC efflux inhibition in WT prostate cells with either reversan or Ko143 leads to enhanced prostate epithelial differentiation, possibly due to an increased accumulation of differentiation signals (eg, retinoic acid, hydrocorticosteroid, testosterone, glutathione, and porphyrins). Activation of steroid receptor signaling and alteration in the redox status may lead to enhanced differentiation. (D) Complete MDR-ABC efflux inhibition in ABCG2 null prostate cells with reversan leads to enhanced prostate epithelial differentiation, possibly due to an increased accumulation of differentiation signals (eg, retinoic acid, hydrocorticosteroid, testosterone, glutathione, and porphyrins). Color images available online at www.liebertpub.com/scd