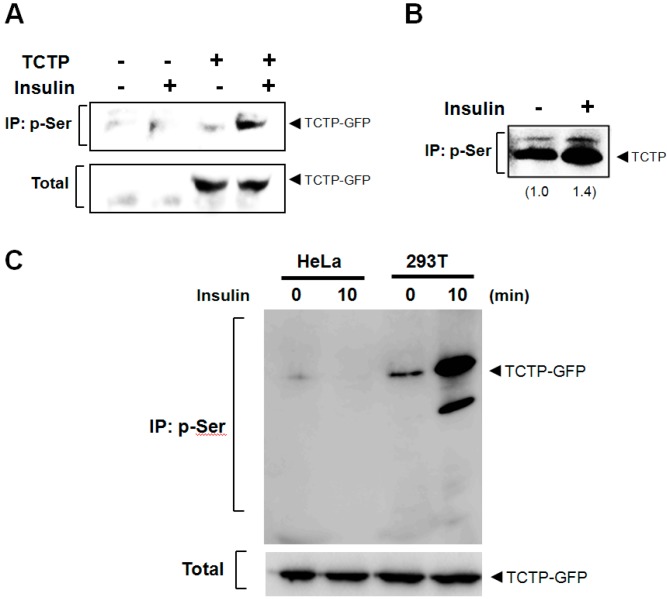
Figure 2.
Insulin-induces phosphorylation of both endogenous and exogenous TCTP. (A) After transfection, the 293T cells to overexpress pEGFP-N1-TCTP construct, insulin was treated at a concentration of 100 nM. Following cytosolic preparation, TCTP phosphorylation at Ser residue(s) was examined through immunoprecipitation using anti-p-Ser antibody, followed by immunoblotting using anti-GFP antibody; (B) Endogenous TCTP phosphorylation at Ser residue following 100 nM insulin treatments in cytosolic fraction was determined by immunoprecipitation with anti-p-Ser antibody and Western blotting with anti-TCTP-specific antibody. Relative band intensities are calculated by Image J software (National Institute of Health, Bethesda, MD, USA) and are expressed as a fold increase of untreated cells; (C) Cells were transfected with pEGFP-N1-TCTP construct and were treated with 100 nM insulin for 10 min in HeLa and 293T cells, respectively. After cytosolic preparation, phosphorylated TCTP was detected by immunoprecipitation using anti-p-Ser antibodies and immunoblotting with anti-GFP antibody.