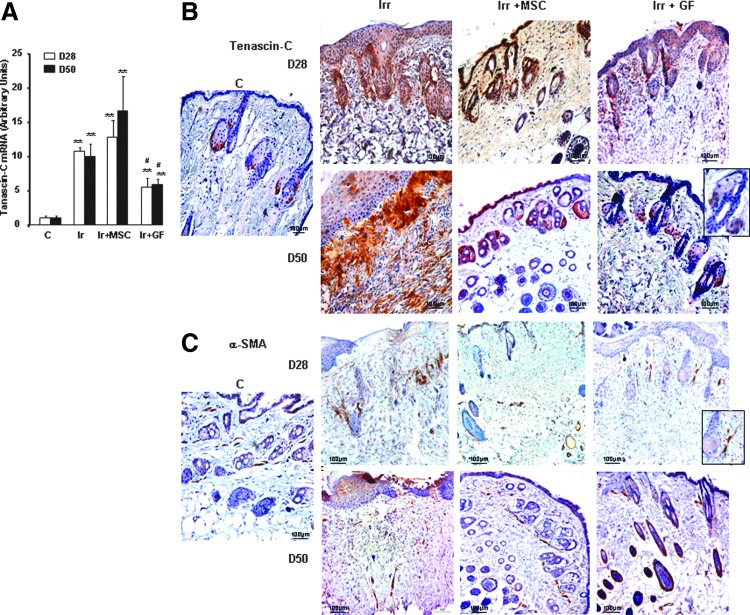
FIG. 4.
Expression of ECM components. (A) Real-time PCR of tenascin-C in nonirradiated control skin, irradiated-untreated skin, and MSC- or GF-treated skin on days 28 and 50 after irradiation. Data are reported relative to control mice and normalized to GAPDH. (B) Representative immunohistochemical staining with the primary anti-tenascin-C antibody and (C) the primary α-SMA antibody. Inserts were amplified local areas from the same images. Original magnification ×20. Results are expressed as mean±SEM. P values were calculated by ANOVA with Bonferroni correction, **P<0.001; compared with nonirradiated controls; #P<0.01 compared with irradiated-untreated controls.