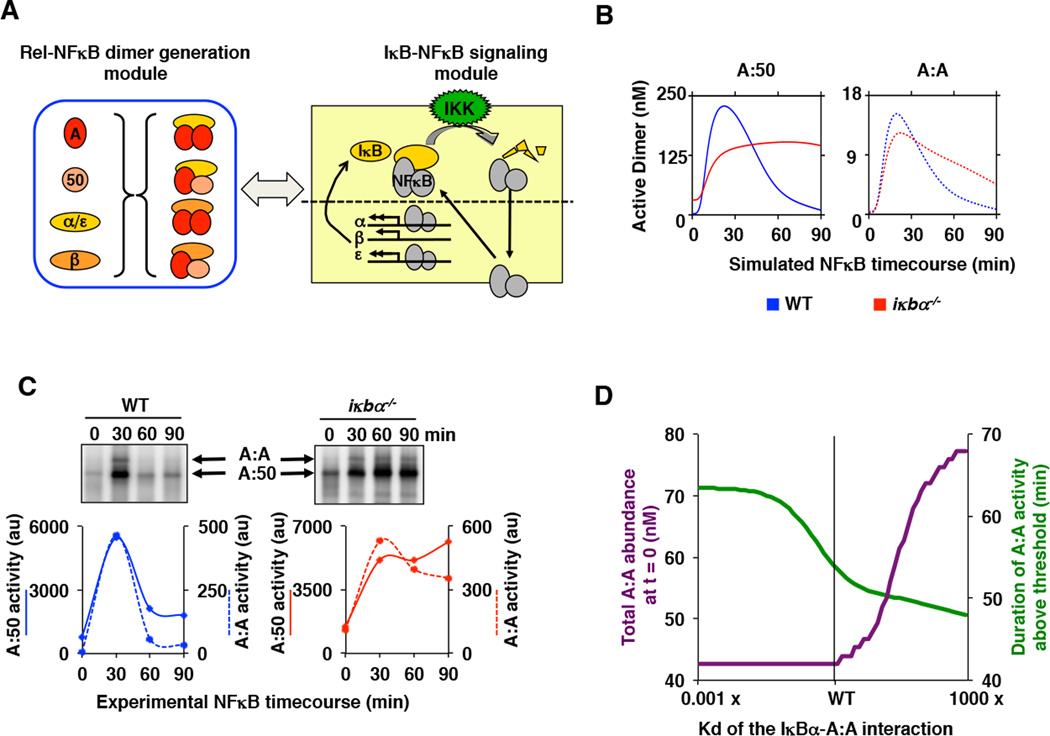
Figure 7. While IκBβ regulates NFκB/RelA homodimer generation, IκBα regulates its signaling.
A. A schematic showing the linking of the Rel-NFκB dimer generation module and the IκB-NFκB signaling module (20) to simulate stimulus-induced activation of multiple NFκB dimers. IκBα, IκBβ, and IκBε interact with A:A and A:50 dimers as described in Figure 5A. A detailed model schematic is shown in Supplemental Figure 5A.
B. Computational time course simulations of TNF-stimulated nuclear DNA binding activity of the indicated NFκB dimers A:50 (left, solid lines) and A:A (right, dashed lines) in wild type (blue) and IκBα-deficient (red) MEFs. Both dimer activities exhibit post-induction repression mediated by IκBα.
C. Electrophoretic mobility shift assays (top panels) of nuclear extracts of wild-type and IκBα-deficient MEFs prepared from TNF-stimulated cells at 30, 60 and 90 minutes. Quantitation (bottom panels) of the A:50 (solid lines) A:A and A:50 (dashed lines) NFκB dimers show prolonged activation in IκBα deficient (right, red) as compared to wild-type (left, blue) MEFs. Shown is a representative of at least three independent experiments.
D. Computational simulations of A:A abundance (green) and duration of TNF-induced A:A activity (purple) as a function of the interaction affinity between IκBα and the A:A. These simulations were performed with the integrated model described in (A), and they show that a lower Kd would allow IκBα to contribute to A:A generation, while a higher Kd would reduce IκBα’s ability to terminate TNF-induced A:A activity.