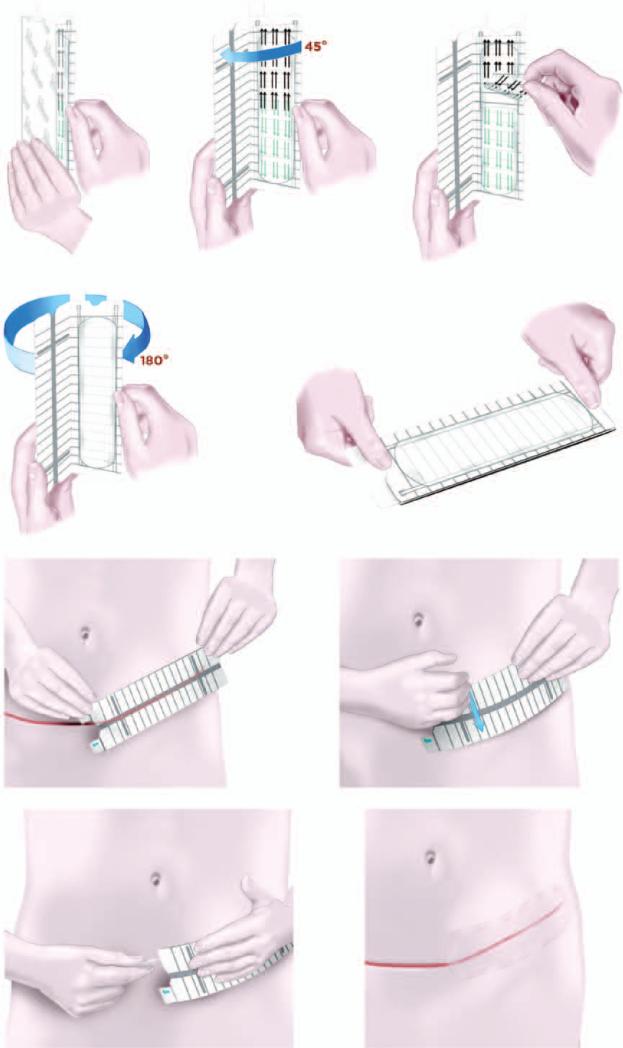
Fig. 1.
Schematic depiction of the embrace device. (Above, left) The embrace device used in the clinical trial is a 16x5-cm silicone elastomeric dressing that adheres to the skin using a pressure-sensitive silicone adhesive. (Above, center) The user initially opens the applicator approximately 45 degrees and (above, right) the protective liner is peeled back from the adhesive dressing. (Second row, left) The applicator is then fully opened 180 degrees to strain the dressing. (Second row, right) Once fully opened, the device is ready for application. (Third row, left) The dressing is applied directly over the center of the closed incision, and (third row, right) the user firmly presses or rubs the applicator to activate the adhesive. (Below, left) Tabs at the end of the applicator are pulled away to release the dressing and the applicator is discarded, (below, right) after which the dressing remains on for approximately 7 days.