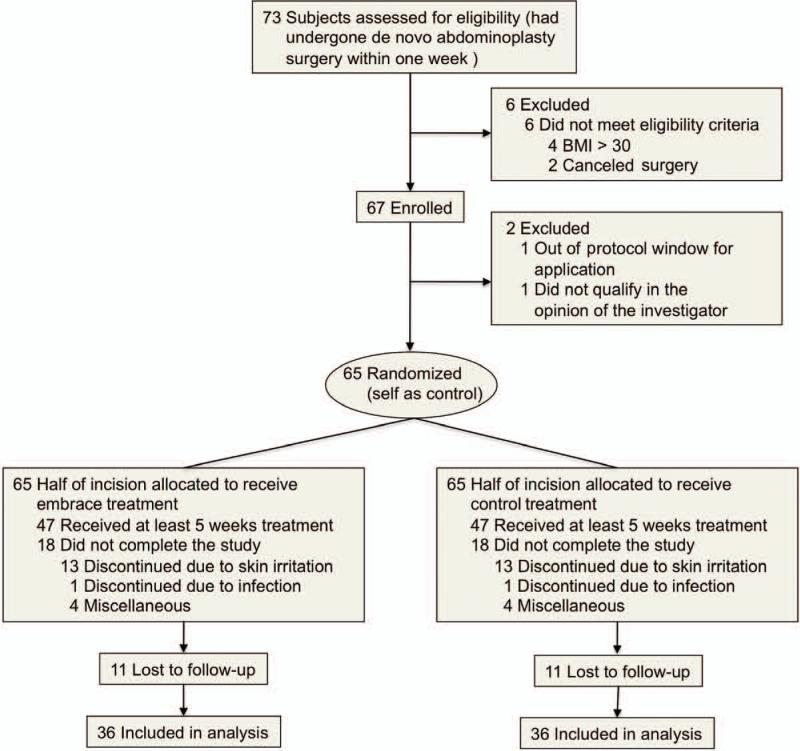
Fig. 2.
Flow diagram of clinical trial. Seventy-three subjects were initially assessed for eligibility, with four excluded because of a body mass index (BMI) in excess of 30 and two excluded after canceling surgery. In addition, two were excluded after enrollment but before treatment (one because of a body mass index out of range and the other as a result of missing the treatment window). A total of 65 subjects underwent randomization (self as control), with half of each incision allocated to receive either embrace or control treatment. Eighteen subjects did not complete the study, with 13 discontinuing because of skin irritation, one because of a wound-site infection, and four for miscellaneous reasons. An additional 11 subjects completed treatment but were subsequently lost to follow-up, resulting in 36 subjects included in the final analysis.